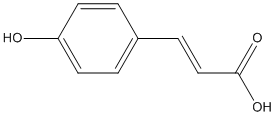
p-coumaric-acid
General
Type : Feruloyl || Hydroxycinnamic-ester || Acrylate
Chemical_Nomenclature : (E)-3-(4-hydroxyphenyl)prop-2-enoic acid
Canonical SMILES : C1=CC(=CC=C1C=CC(=O)O)O
InChI : InChI=1S\/C9H8O3\/c10-8-4-1-7(2-5-8)3-6-9(11)12\/h1-6,10H,(H,11,12)\/b6-3+
InChIKey : NGSWKAQJJWESNS-ZZXKWVIFSA-N
Other name(s) : 4-Hydroxycinnamic acid, p-coumaric acid, p-Hydroxycinnamic acid, 4-Coumaric acid, CHEBI:32374, CHEBI:36090
Target
Families : Tannase, Esterase_phb
References (5)
| Title : Crystal structure of the Fusarium oxysporum tannase-like feruloyl esterase FaeC in complex with p-coumaric acid provides insight into ligand binding - Ferousi_2023_FEBS.Lett_597_1415 |
| Author(s) : Ferousi C , Kosinas C , Nikolaivits E , Topakas E , Dimarogona M |
| Ref : FEBS Letters , 597 :1415 , 2023 |
| Abstract : Ferousi_2023_FEBS.Lett_597_1415 |
| ESTHER : Ferousi_2023_FEBS.Lett_597_1415 |
| PubMedSearch : Ferousi_2023_FEBS.Lett_597_1415 |
| PubMedID: 36961270 |
| Gene_locus related to this paper: fusox-a0a1d3s5h0 |
| Title : Reducing cell wall feruloylation by expression of a fungal ferulic acid esterase in Festuca arundinacea modifies plant growth, leaf morphology and the turnover of cell wall arabinoxylans - de O Buanafina_2017_PLoS.One_12_e0185312 |
| Author(s) : de O Buanafina MM , Iyer PR , Buanafina MF , Shearer EA |
| Ref : PLoS ONE , 12 :e0185312 , 2017 |
| Abstract : de O Buanafina_2017_PLoS.One_12_e0185312 |
| ESTHER : de O Buanafina_2017_PLoS.One_12_e0185312 |
| PubMedSearch : de O Buanafina_2017_PLoS.One_12_e0185312 |
| PubMedID: 28934356 |
| Gene_locus related to this paper: aspni-FAEA |
| Title : Characterization of a feruloyl esterase B from Talaromyces cellulolyticus - Watanabe_2015_Biosci.Biotechnol.Biochem_79_1845 |
| Author(s) : Watanabe M , Yoshida E , Fukada H , Inoue H , Tokura M , Ishikawa K |
| Ref : Biosci Biotechnol Biochem , 79 :1845 , 2015 |
| Abstract : Watanabe_2015_Biosci.Biotechnol.Biochem_79_1845 |
| ESTHER : Watanabe_2015_Biosci.Biotechnol.Biochem_79_1845 |
| PubMedSearch : Watanabe_2015_Biosci.Biotechnol.Biochem_79_1845 |
| PubMedID: 26110915 |
| Gene_locus related to this paper: talce-faeB |
| Title : Effects of enzymatic removal of plant cell wall acylation (acetylation, p-coumaroylation, and feruloylation) on accessibility of cellulose and xylan in natural (non-pretreated) sugar cane fractions - Varnai_2014_Biotechnol.Biofuels_7_153 |
| Author(s) : Varnai A , Costa TH , Faulds CB , Milagres AM , Siika-aho M , Ferraz A |
| Ref : Biotechnol Biofuels , 7 :153 , 2014 |
| Abstract : Varnai_2014_Biotechnol.Biofuels_7_153 |
| ESTHER : Varnai_2014_Biotechnol.Biofuels_7_153 |
| PubMedSearch : Varnai_2014_Biotechnol.Biofuels_7_153 |
| PubMedID: 25328538 |
| Title : Enzymatic synthesis of hydroxycinnamic acid glycerol esters using type A feruloyl esterase from Aspergillus niger - Tsuchiyama_2007_Biosci.Biotechnol.Biochem_71_2606 |
| Author(s) : Tsuchiyama M , Sakamoto T , Tanimori S , Murata S , Kawasaki H |
| Ref : Biosci Biotechnol Biochem , 71 :2606 , 2007 |
| Abstract : Tsuchiyama_2007_Biosci.Biotechnol.Biochem_71_2606 |
| ESTHER : Tsuchiyama_2007_Biosci.Biotechnol.Biochem_71_2606 |
| PubMedSearch : Tsuchiyama_2007_Biosci.Biotechnol.Biochem_71_2606 |
| PubMedID: 17928681 |