gamma-Valerolactone
General
Type : Lactone
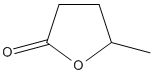
Chemical_Nomenclature : 5-methyloxolan-2-one
Canonical SMILES : CC1CCC(=O)O1
InChI : InChI=1S\/C5H8O2\/c1-4-2-3-5(6)7-4\/h4H,2-3H2,1H3
InChIKey : GAEKPEKOJKCEMS-UHFFFAOYSA-N
Other name(s) : 4-Valerolactone, 4-Pentanolide, 4-Hydroxypentanoic acid lactone, Gamma-Pentalactone
Target
Families : No family
References (3)
| Title : Insights into the Lactonase Mechanism of Serum Paraoxonase 1 (PON1): Experimental and Quantum Mechanics\/Molecular Mechanics (QM\/MM) Studies - Le_2015_J.Phys.Chem.B_119_9571 |
| Author(s) : Le QA , Kim S , Chang R , Kim YH |
| Ref : J Phys Chem B , 119 :9571 , 2015 |
| Abstract : Le_2015_J.Phys.Chem.B_119_9571 |
| ESTHER : Le_2015_J.Phys.Chem.B_119_9571 |
| PubMedSearch : Le_2015_J.Phys.Chem.B_119_9571 |
| PubMedID: 26146888 |
| Title : A chemo-enzymatic route to synthesize (S)-gamma-valerolactone from levulinic acid - Gotz_2013_Appl.Microbiol.Biotechnol_97_3865 |
| Author(s) : Gotz K , Liese A , Ansorge-Schumacher M , Hilterhaus L |
| Ref : Applied Microbiology & Biotechnology , 97 :3865 , 2013 |
| Abstract : Gotz_2013_Appl.Microbiol.Biotechnol_97_3865 |
| ESTHER : Gotz_2013_Appl.Microbiol.Biotechnol_97_3865 |
| PubMedSearch : Gotz_2013_Appl.Microbiol.Biotechnol_97_3865 |
| PubMedID: 23296499 |