epsilon-caprolactone
General
Type : Lactone || Ring opening polymerization substrate
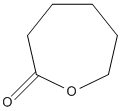
Chemical_Nomenclature : oxepan-2-one
Canonical SMILES : C1CCC(=O)OCC1
InChI : InChI=1S\/C6H10O2\/c7-6-4-2-1-3-5-8-6\/h1-5H2
InChIKey : PAPBSGBWRJIAAV-UHFFFAOYSA-N
Other name(s) : oxepan-2-one, 6-Hexanolactone, Epsilon caprolactone, 2-Oxepanone, Caprolactone, ECE, CHEMBL373123, SCHEMBL10850, CHEBI:17915, ZINC388417
Target
Families : Canar_LipB
References (6)
| Title : Comparison of Candida antarctica Lipase B Variants for Conversion of epsilon-Caprolactone in Aqueous Medium-Part 2 - Hock_2018_Polymers.(Basel)_10_524 |
| Author(s) : Hock H , Engel S , Weingarten S , Keul H , Schwaneberg U , Moller M , Bocola M |
| Ref : Polymers (Basel) , 10 : , 2018 |
| Abstract : Hock_2018_Polymers.(Basel)_10_524 |
| ESTHER : Hock_2018_Polymers.(Basel)_10_524 |
| PubMedSearch : Hock_2018_Polymers.(Basel)_10_524 |
| PubMedID: 30966558 |
| Gene_locus related to this paper: canar-LipB |
| Title : Lipase-mediated direct in situ ring-opening polymerization of E-caprolactone formed by a chemo-enzymatic method - Zhang_2018_J.Biotechnol_281_74 |
| Author(s) : Zhang Y , Lu P , Sun Q , Li T , Zhao L , Gao X , Wang F , Liu J |
| Ref : J Biotechnol , 281 :74 , 2018 |
| Abstract : Zhang_2018_J.Biotechnol_281_74 |
| ESTHER : Zhang_2018_J.Biotechnol_281_74 |
| PubMedSearch : Zhang_2018_J.Biotechnol_281_74 |
| PubMedID: 29908204 |
| Title : CaLB Catalyzed Conversion of sigma-Caprolactone in Aqueous Medium. Part 1: Immobilization of CaLB to Microgels - Engel_2016_Polymers.(Basel)_8_372 |
| Author(s) : Engel S , Hock H , Bocola M , Keul H , Schwaneberg U , Moller M |
| Ref : Polymers (Basel) , 8 : , 2016 |
| Abstract : Engel_2016_Polymers.(Basel)_8_372 |
| ESTHER : Engel_2016_Polymers.(Basel)_8_372 |
| PubMedSearch : Engel_2016_Polymers.(Basel)_8_372 |
| PubMedID: 30974648 |
| Title : Lipase-catalyzed ring-opening copolymerization of sigma-caprolactone and beta-lactam - Stavila_2014_Biomacromolecules_15_234 |
| Author(s) : Stavila E , Alberda van Ekenstein GO , Woortman AJ , Loos K |
| Ref : Biomacromolecules , 15 :234 , 2014 |
| Abstract : Stavila_2014_Biomacromolecules_15_234 |
| ESTHER : Stavila_2014_Biomacromolecules_15_234 |
| PubMedSearch : Stavila_2014_Biomacromolecules_15_234 |
| PubMedID: 24294825 |
| Title : Modeling enzymatic kinetic pathways for ring-opening lactone polymerization - Johnson_2011_Biomacromolecules_12_3337 |
| Author(s) : Johnson PM , Kundu S , Beers KL |
| Ref : Biomacromolecules , 12 :3337 , 2011 |
| Abstract : Johnson_2011_Biomacromolecules_12_3337 |
| ESTHER : Johnson_2011_Biomacromolecules_12_3337 |
| PubMedSearch : Johnson_2011_Biomacromolecules_12_3337 |
| PubMedID: 21834510 |
| Title : Enzymatic Ring-Opening Polymerization of Lactones Catalyzed by Lipase - Uyama_1993_Chem.Lett_7_1149 |
| Author(s) : Uyama H , Kobayashi S |
| Ref : Chem Lett , 22 :1149 , 1993 |
| Abstract : Uyama_1993_Chem.Lett_7_1149 |
| ESTHER : Uyama_1993_Chem.Lett_7_1149 |
| PubMedSearch : Uyama_1993_Chem.Lett_7_1149 |
| PubMedID: |