Z11-16Ac
General
Type : Odorant || Pheromone || Acetate
Chemical_Nomenclature : [(Z)-hexadec-11-enyl] acetate
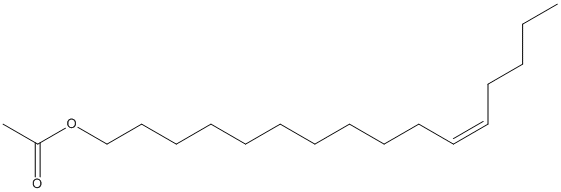
Canonical SMILES : CCCCC=CCCCCCCCCCCOC(=O)C
InChI : InChI=1S\/C18H34O2\/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-18(2)19\/h6-7H,3-5,8-17H2,1-2H3\/b7-6-
InChIKey : BTKXLQSCEOHKTF-SREVYHEPSA-N
Other name(s) : (Z)-11-Hexadecenyl acetate, (Z)-11-Hexadecen-1-yl acetate, (Z)-Hexadec-11-en-1-yl acetate, [(Z)-hexadec-11-enyl] acetate, Z11-16:OAc, SCHEMBL434978, ZINC33840850
Target
Families : Carb_B_Arthropoda
References (5)
| Title : Two carboxylesterase genes in Plutella xylostella associated with sex pheromones and plant volatiles degradation - Wang_2021_Pest.Manag.Sci__ |
| Author(s) : Wang MM , Long GJ , Guo H , Liu XZ , Wang H , Dewer Y , Li ZQ , Liu K , Zhang QL , Ma YF , He P , He M |
| Ref : Pest Manag Sci , : , 2021 |
| Abstract : Wang_2021_Pest.Manag.Sci__ |
| ESTHER : Wang_2021_Pest.Manag.Sci__ |
| PubMedSearch : Wang_2021_Pest.Manag.Sci__ |
| PubMedID: 33527628 |
| Gene_locus related to this paper: pluxy-CCE016a , pluxy-CCE016c |
| Title : Identification and Field Evaluation of the Sex Pheromone of Orthaga achatina (Lepidoptera: Pyralidae) - Yan_2018_J.Chem.Ecol_44_886 |
| Author(s) : Yan Q , Li HD , Chen Y , Ye ZF , You XY , Zhou J , Mu LF , Liu SJ , Kong XB , Khuhro SA , Dong SL |
| Ref : J Chem Ecol , 44 :886 , 2018 |
| Abstract : Yan_2018_J.Chem.Ecol_44_886 |
| ESTHER : Yan_2018_J.Chem.Ecol_44_886 |
| PubMedSearch : Yan_2018_J.Chem.Ecol_44_886 |
| PubMedID: 30094705 |
| Title : Response of Mythimna unipuncta males to components of the Sesamia nonagrioides pheromone - Eizaguirre_2009_J.Chem.Ecol_35_779 |
| Author(s) : Eizaguirre M , Lopez C , Sans A , Bosch D , Albajes R |
| Ref : J Chem Ecol , 35 :779 , 2009 |
| Abstract : Eizaguirre_2009_J.Chem.Ecol_35_779 |
| ESTHER : Eizaguirre_2009_J.Chem.Ecol_35_779 |
| PubMedSearch : Eizaguirre_2009_J.Chem.Ecol_35_779 |
| PubMedID: 19593653 |
| Title : Aquatic ecotoxicity of a pheromonal antagonist in Daphnia magna and Desmodesmus subspicatus - Rosa_2006_Aquat.Toxicol_79_296 |
| Author(s) : Rosa E , Barata C , Damasio J , Bosch MP , Guerrero A |
| Ref : Aquat Toxicol , 79 :296 , 2006 |
| Abstract : Rosa_2006_Aquat.Toxicol_79_296 |
| ESTHER : Rosa_2006_Aquat.Toxicol_79_296 |
| PubMedSearch : Rosa_2006_Aquat.Toxicol_79_296 |
| PubMedID: 16899308 |
| Title : Chemical communication in heliothine moths. VIL Correlation between diminished responses to point-source plumes and single filaments similarly tainted with a behavioral antagonist - Vickers_1997_J.Comp.Physiol.A_180_523 |
| Author(s) : Vickers NJ , Baker TC |
| Ref : J Comp Physiol A , 180 :523 , 1997 |
| Abstract : Vickers_1997_J.Comp.Physiol.A_180_523 |
| ESTHER : Vickers_1997_J.Comp.Physiol.A_180_523 |
| PubMedSearch : Vickers_1997_J.Comp.Physiol.A_180_523 |
| PubMedID: |