Yoshimulactone-Green
General
Type : Lactone || Strigolactone receptors ligand || Fluorescent Probe || Benzoate || Xanthene
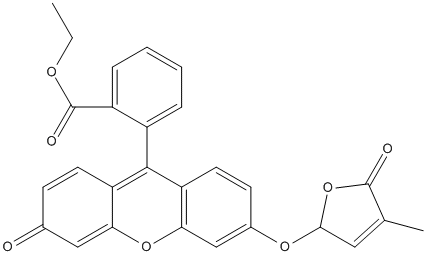
Chemical_Nomenclature : ethyl 2-[3-[(4-methyl-5-oxo-2H-furan-2-yl)oxy]-6-oxoxanthen-9-yl]benzoate
Canonical SMILES : CCOC(=O)C1=CC=CC=C1C2=C3C=CC(=O)C=C3OC4=C2C=CC(=C4)OC5C=C(C(=O)O5)C
InChI : InChI=1S\/C27H20O7\/c1-3-31-27(30)19-7-5-4-6-18(19)25-20-10-8-16(28)13-22(20)33-23-14-17(9-11-21(23)25)32-24-12-15(2)26(29)34-24\/h4-14,24H,3H2,1-2H3
InChIKey : CHRFZINIRYDHJV-UHFFFAOYSA-N
Other name(s) : Yoshimulactone Green (YLG), YLG, E1238, Ethyl 2-[6-[(2,5-Dihydro-4-methyl-5-oxo-2-furanyl)oxy]-3-oxo-3H-xanthen-9-yl]benzoate, 2-[6-[(2,5-Dihydro-4-methyl-5-oxo-2-furanyl)oxy]-3-oxo-3H-xanthen-9-yl]benzoic Acid Ethyl Ester
Target
Families : RsbQ-like, Plant_carboxylesterase
References (8)
| Title : Perception of butenolides by Bacillus subtilis via the alpha\/beta hydrolase RsbQ - Melville_2023_Curr.Biol__ |
| Author(s) : Melville KT , Kamran M , Yao J , Costa M , Holland M , Taylor NL , Fritz G , Flematti GR , Waters MT |
| Ref : Current Biology , : , 2023 |
| Abstract : Melville_2023_Curr.Biol__ |
| ESTHER : Melville_2023_Curr.Biol__ |
| PubMedSearch : Melville_2023_Curr.Biol__ |
| PubMedID: 38183985 |
| Gene_locus related to this paper: bacsu-RsbQ |
| Title : Expansion of the Strigolactone Profluorescent Probes Repertory: The Right Probe for the Right Application - de Saint Germain_2022_Front.Plant.Sci_13_887347 |
| Author(s) : de Saint Germain A , Clave G , Schouveiler P , Pillot JP , Singh AV , Chevalier A , Daignan Fornier S , Guillory A , Bonhomme S , Rameau C , Boyer FD |
| Ref : Front Plant Sci , 13 :887347 , 2022 |
| Abstract : de Saint Germain_2022_Front.Plant.Sci_13_887347 |
| ESTHER : de Saint Germain_2022_Front.Plant.Sci_13_887347 |
| PubMedSearch : de Saint Germain_2022_Front.Plant.Sci_13_887347 |
| PubMedID: 35720613 |
| Title : Catabolism of strigolactones by a carboxylesterase - Xu_2021_Nat.Plants_7_1495 |
| Author(s) : Xu E , Chai L , Zhang S , Yu R , Zhang X , Xu C , Hu Y |
| Ref : Nat Plants , 7 :1495 , 2021 |
| Abstract : Xu_2021_Nat.Plants_7_1495 |
| ESTHER : Xu_2021_Nat.Plants_7_1495 |
| PubMedSearch : Xu_2021_Nat.Plants_7_1495 |
| PubMedID: 34764442 |
| Gene_locus related to this paper: arath-CXE15 |
| Title : Discovery of a Broad-Spectrum Fluorogenic Agonist for Strigolactone Receptors through a Computational Approach - Wang_2021_J.Agric.Food.Chem__ |
| Author(s) : Wang DW , Yu SY , Pang ZL , Ma DJ , Liang L , Wang X , Wei T , Yang HZ , Ma YQ , Xi Z |
| Ref : Journal of Agricultural and Food Chemistry , : , 2021 |
| Abstract : Wang_2021_J.Agric.Food.Chem__ |
| ESTHER : Wang_2021_J.Agric.Food.Chem__ |
| PubMedSearch : Wang_2021_J.Agric.Food.Chem__ |
| PubMedID: 34478295 |
| Title : Triazole Ureas Covalently Bind to Strigolactone Receptor and Antagonize Strigolactone Responses - Nakamura_2019_Mol.Plant_12_44 |
| Author(s) : Nakamura H , Hirabayashi K , Miyakawa T , Kikuzato K , Hu W , Xu Y , Jiang K , Takahashi I , Niiyama R , Dohmae N , Tanokura M , Asami T |
| Ref : Mol Plant , 12 :44 , 2019 |
| Abstract : Nakamura_2019_Mol.Plant_12_44 |
| ESTHER : Nakamura_2019_Mol.Plant_12_44 |
| PubMedSearch : Nakamura_2019_Mol.Plant_12_44 |
| PubMedID: 30391752 |
| Gene_locus related to this paper: orysj-Q10QA5 |
| Title : An allelic series at the KARRIKIN INSENSITIVE 2 locus of Arabidopsis thaliana decouples ligand hydrolysis and receptor degradation from downstream signalling - Yao_2018_Plant.J_96_75 |
| Author(s) : Yao J , Mashiguchi K , Scaffidi A , Akatsu T , Melville KT , Morita R , Morimoto Y , Smith SM , Seto Y , Flematti GR , Yamaguchi S , Waters MT |
| Ref : Plant J , 96 :75 , 2018 |
| Abstract : Yao_2018_Plant.J_96_75 |
| ESTHER : Yao_2018_Plant.J_96_75 |
| PubMedSearch : Yao_2018_Plant.J_96_75 |
| PubMedID: 29982999 |
| Gene_locus related to this paper: arath-KAI2.D14L |
| Title : Discovery of Shoot Branching Regulator Targeting Strigolactone Receptor DWARF14 - Yoshimura_2018_ACS.Cent.Sci_4_230 |
| Author(s) : Yoshimura M , Sato A , Kuwata K , Inukai Y , Kinoshita T , Itami K , Tsuchiya Y , Hagihara S |
| Ref : ACS Cent Sci , 4 :230 , 2018 |
| Abstract : Yoshimura_2018_ACS.Cent.Sci_4_230 |
| ESTHER : Yoshimura_2018_ACS.Cent.Sci_4_230 |
| PubMedSearch : Yoshimura_2018_ACS.Cent.Sci_4_230 |
| PubMedID: 29532023 |
| Title : PARASITIC PLANTS. Probing strigolactone receptors in Striga hermonthica with fluorescence - Tsuchiya_2015_Science_349_864 |
| Author(s) : Tsuchiya Y , Yoshimura M , Sato Y , Kuwata K , Toh S , Holbrook-Smith D , Zhang H , McCourt P , Itami K , Kinoshita T , Hagihara S |
| Ref : Science , 349 :864 , 2015 |
| Abstract : Tsuchiya_2015_Science_349_864 |
| ESTHER : Tsuchiya_2015_Science_349_864 |
| PubMedSearch : Tsuchiya_2015_Science_349_864 |
| PubMedID: 26293962 |
| Gene_locus related to this paper: strhe-ShHTL2 , strhe-ShHTL10 , strhe-ShHTL6 , strhe-ShHTL9 , strhe-ShHTL8 , strhe-ShHTL11 , strhe-ShD14 , strhe-ShHTL4 , strhe-ShHTL1 , strhe-ShHTL7 , strhe-ShHTL3 , strhe-ShHTL5 |