Vinyl-stearate
General
Type : Vinyl alkyl ester || Alkyl ester || Stearate || Water-insoluble medium and long chain ester
Chemical_Nomenclature : ethenyl octadecanoate
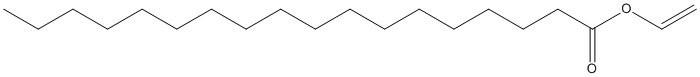
Canonical SMILES : CCCCCCCCCCCCCCCCCC(=O)OC=C
InChI : InChI=1S\/C20H38O2\/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22-4-2\/h4H,2-3,5-19H2,1H3
InChIKey : AFSIMBWBBOJPJG-UHFFFAOYSA-N
Other name(s) : Vinyl stearate, Vinylstearate, Octadecanoic acid, ethenyl ester, Stearic acid, vinyl ester, UNII-F6L6TFV7BX, Ethenyl octadecanoate
Target
Families : No family
References (4)
| Title : Lipases efficiently stearate and cutinases acetylate the surface of arabinoxylan films - Stepan_2013_J.Biotechnol_167_16 |
| Author(s) : Stepan AM , Anasontzis GE , Matama T , Cavaco-Paulo A , Olsson L , Gatenholm P |
| Ref : J Biotechnol , 167 :16 , 2013 |
| Abstract : Stepan_2013_J.Biotechnol_167_16 |
| ESTHER : Stepan_2013_J.Biotechnol_167_16 |
| PubMedSearch : Stepan_2013_J.Biotechnol_167_16 |
| PubMedID: 23774036 |
| Title : Direct enzymatic acylation of cellulose pretreated in BMIMCl ionic liquid - Gremos_2011_Bioresour.Technol_102_1378 |
| Author(s) : Gremos S , Zarafeta D , Kekos D , Kolisis F |
| Ref : Bioresour Technol , 102 :1378 , 2011 |
| Abstract : Gremos_2011_Bioresour.Technol_102_1378 |
| ESTHER : Gremos_2011_Bioresour.Technol_102_1378 |
| PubMedSearch : Gremos_2011_Bioresour.Technol_102_1378 |
| PubMedID: 20888759 |
| Title : A spectrophotometric transesterification-based assay for lipases in organic solvent - Goujard_2009_Anal.Biochem_385_161 |
| Author(s) : Goujard L , Villeneuve P , Barea B , Lecomte J , Pina M , Claude S , Le Petit J , Ferre E |
| Ref : Analytical Biochemistry , 385 :161 , 2009 |
| Abstract : Goujard_2009_Anal.Biochem_385_161 |
| ESTHER : Goujard_2009_Anal.Biochem_385_161 |
| PubMedSearch : Goujard_2009_Anal.Biochem_385_161 |
| PubMedID: 19013125 |
| Title : Modification of cellulose fiber surfaces by use of a lipase and a xyloglucan endotransglycosylase - Gustavsson_2005_Biomacromolecules_6_196 |
| Author(s) : Gustavsson MT , Persson PV , Iversen T , Martinelle M , Hult K , Teeri TT , Brumer H, 3rd |
| Ref : Biomacromolecules , 6 :196 , 2005 |
| Abstract : Gustavsson_2005_Biomacromolecules_6_196 |
| ESTHER : Gustavsson_2005_Biomacromolecules_6_196 |
| PubMedSearch : Gustavsson_2005_Biomacromolecules_6_196 |
| PubMedID: 15638521 |