Vinyl-crotonate
General
Type : Vinyl alkenyl ester || Alkenyl ester
Chemical_Nomenclature : ethenyl (E)-but-2-enoate
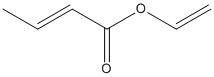
Canonical SMILES :
InChI : InChI=1S\/C6H8O2\/c1-3-5-6(7)8-4-2\/h3-5H,2H2,1H3\/b5-3+
InChIKey : IYNRVIKPUTZSOR-HWKANZROSA-N
Other name(s) : Vinyl crotonate, Vinyl 2-butenoate, Crotonic acid vinyl ester, Crotonic acid, vinyl ester, Ethenyl (E)-but-2-enoate
Target
Families : No family
References (2)
| Title : Efficient regioselective synthesis of 3'-O-crotonylfloxuridine catalysed by Pseudomonas cepacia lipase - Zhao_2009_Biotechnol.Appl.Biochem_52_45 |
| Author(s) : Zhao Z , Zong M , Li N |
| Ref : Biotechnol Appl Biochem , 52 :45 , 2009 |
| Abstract : Zhao_2009_Biotechnol.Appl.Biochem_52_45 |
| ESTHER : Zhao_2009_Biotechnol.Appl.Biochem_52_45 |
| PubMedSearch : Zhao_2009_Biotechnol.Appl.Biochem_52_45 |
| PubMedID: 18373494 |