Vinyl-acrylate
General
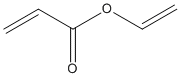
Type : Vinyl alkenyl ester || Alkenyl ester || Acrylate
Chemical_Nomenclature : ethenyl prop-2-enoate
Canonical SMILES : C=CC(=O)OC=C
InChI : InChI=1S\/C5H6O2\/c1-3-5(6)7-4-2\/h3-4H,1-2H2
InChIKey : BLCTWBJQROOONQ-UHFFFAOYSA-N
Other name(s) : Vinyl acrylate, 2-Propenoic acid, ethenyl ester, Acrylic acid, vinyl ester, Ethenyl 2-propenoate, Ethenyl prop-2-enoate
Target
Families : No family
References (2)
| Title : Optimization of lipase-catalyzed synthesis of sorbitan acrylate using response surface methodology - Jeong_2007_Appl.Biochem.Biotechnol_137-140_595 |
| Author(s) : Jeong GT , Park DH |
| Ref : Appl Biochem Biotechnol , 137-140 :595 , 2007 |
| Abstract : Jeong_2007_Appl.Biochem.Biotechnol_137-140_595 |
| ESTHER : Jeong_2007_Appl.Biochem.Biotechnol_137-140_595 |
| PubMedSearch : Jeong_2007_Appl.Biochem.Biotechnol_137-140_595 |
| PubMedID: 18478419 |