VPA-glucuronide
General
Type : Glucuronide
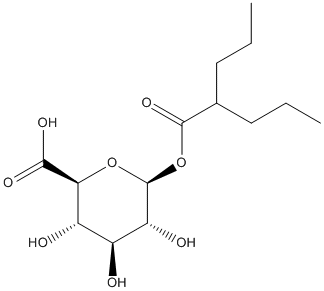
Chemical_Nomenclature : (2S,3S,4S,5R,6S)-3,4,5-trihydroxy-6-(2-propylpentanoyloxy)oxane-2-carboxylic acid
Canonical SMILES : CCCC(CCC)C(=O)OC1C(C(C(C(O1)C(=O)O)O)O)O
InChI : InChI=1S\/C14H24O8\/c1-3-5-7(6-4-2)13(20)22-14-10(17)8(15)9(16)11(21-14)12(18)19\/h7-11,14-17H,3-6H2,1-2H3,(H,18,19)\/t8-,9-,10+,11-,14-\/m0\/s1
InChIKey : XXKSYIHWRBBHIC-JVWRJRKNSA-N
Other name(s) : VPA glucuronide, VPA-G, Valproic acid glucuronide, VPAG, Valproic Acid beta-D-Glucuronide, Valproate glucuronide
Target
Families : ACPH_Peptidase_S9
References (4)
| Title : Identification of valproic acid glucuronide hydrolase as a key enzyme for the interaction of valproic acid with carbapenem antibiotics - Suzuki_2010_Drug.Metab.Dispos_38_1538 |
| Author(s) : Suzuki E , Yamamura N , Ogura Y , Nakai D , Kubota K , Kobayashi N , Miura S , Okazaki O |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1538 , 2010 |
| Abstract : Suzuki_2010_Drug.Metab.Dispos_38_1538 |
| ESTHER : Suzuki_2010_Drug.Metab.Dispos_38_1538 |
| PubMedSearch : Suzuki_2010_Drug.Metab.Dispos_38_1538 |
| PubMedID: 20551238 |
| Title : Characterization of inhibitory effect of carbapenem antibiotics on the deconjugation of valproic acid glucuronide - Masuo_2010_Drug.Metab.Dispos_38_1828 |
| Author(s) : Masuo Y , Ito K , Yamamoto T , Hisaka A , Honma M , Suzuki H |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 38 :1828 , 2010 |
| Abstract : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| ESTHER : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedSearch : Masuo_2010_Drug.Metab.Dispos_38_1828 |
| PubMedID: 20581094 |
| Title : Decreased valproate level caused by VPA-glucuronidase inhibition by carbapenem antibiotics - Nakamura_2008_Drug.Metab.Lett_2_280 |
| Author(s) : Nakamura Y , Nakahira K , Mizutani T |
| Ref : Drug Metab Lett , 2 :280 , 2008 |
| Abstract : Nakamura_2008_Drug.Metab.Lett_2_280 |
| ESTHER : Nakamura_2008_Drug.Metab.Lett_2_280 |
| PubMedSearch : Nakamura_2008_Drug.Metab.Lett_2_280 |
| PubMedID: 19356106 |
| Title : Interaction between valproic acid and carbapenem antibiotics - Mori_2007_Drug.Metab.Rev_39_647 |
| Author(s) : Mori H , Takahashi K , Mizutani T |
| Ref : Drug Metabolism Reviews , 39 :647 , 2007 |
| Abstract : Mori_2007_Drug.Metab.Rev_39_647 |
| ESTHER : Mori_2007_Drug.Metab.Rev_39_647 |
| PubMedSearch : Mori_2007_Drug.Metab.Rev_39_647 |
| PubMedID: 18058328 |