Trihexanoin
General
Type : Acyl-Glycerol || Triacylglycerol || Fatty acid ester || Water-insoluble medium and long chain ester
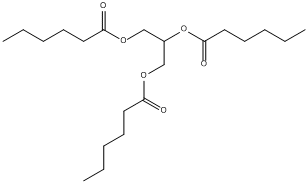
Chemical_Nomenclature : 2,3-di(hexanoyloxy)propyl hexanoate
Canonical SMILES : CCCCCC(=O)OCC(COC(=O)CCCCC)OC(=O)CCCCC
InChI : InChI=1S\/C21H38O6\/c1-4-7-10-13-19(22)25-16-18(27-21(24)15-12-9-6-3)17-26-20(23)14-11-8-5-2\/h18H,4-17H2,1-3H3
InChIKey : MAYCICSNZYXLHB-UHFFFAOYSA-N
Other name(s) : C6-triglyceride, Glycerol trihexanoate, Tricaproin, Caproic triglyceride, Glycerol tricaproate, Glyceryl tricaproate
MW : 386.52
Formula : C21H38O6
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
References (4)
| Title : Protein Engineering of Bacillus thermocatenulatus Lipase via Deletion of the alpha5 Helix - Goodarzi_2014_Appl.Biochem.Biotechnol_174_339 |
| Author(s) : Goodarzi N , Karkhane AA , Mirlohi A , Tabandeh F , Torktas I , Aminzadeh S , Yakhchali B , Shamsara M , Ghafouri MA |
| Ref : Appl Biochem Biotechnol , 174 :339 , 2014 |
| Abstract : Goodarzi_2014_Appl.Biochem.Biotechnol_174_339 |
| ESTHER : Goodarzi_2014_Appl.Biochem.Biotechnol_174_339 |
| PubMedSearch : Goodarzi_2014_Appl.Biochem.Biotechnol_174_339 |
| PubMedID: 25064134 |
| Gene_locus related to this paper: bactc-lipas |
| Title : Thermostable lipases from the extreme thermophilic anaerobic bacteria Thermoanaerobacter thermohydrosulfuricus SOL1 and Caldanaerobacter subterraneus subsp. tengcongensis - Royter_2009_Extremophiles_13_769 |
| Author(s) : Royter M , Schmidt M , Elend C , Hobenreich H , Schafer T , Bornscheuer UT , Antranikian G |
| Ref : Extremophiles , 13 :769 , 2009 |
| Abstract : Royter_2009_Extremophiles_13_769 |
| ESTHER : Royter_2009_Extremophiles_13_769 |
| PubMedSearch : Royter_2009_Extremophiles_13_769 |
| PubMedID: 19579003 |
| Gene_locus related to this paper: theet-q3ch51 , thete-TTE1809 |
| Title : Fatty acid selectivity of lipases during acidolysis reaction between oleic acid and monoacid triacylglycerols - Karabulut_2009_J.Agric.Food.Chem_57_10466 |
| Author(s) : Karabulut I , Durmaz G , Hayaloglu AA |
| Ref : Journal of Agricultural and Food Chemistry , 57 :10466 , 2009 |
| Abstract : Karabulut_2009_J.Agric.Food.Chem_57_10466 |
| ESTHER : Karabulut_2009_J.Agric.Food.Chem_57_10466 |
| PubMedSearch : Karabulut_2009_J.Agric.Food.Chem_57_10466 |
| PubMedID: 19835376 |
| Gene_locus related to this paper: humla-1lipa |
| Title : Effect of taurodeoxycholate, colipase and temperature on the interfacial inactivation of porcine pancreatic lipase - Granon_1980_Eur.J.Biochem_111_117 |
| Author(s) : Granon S , Semeriva M |
| Ref : European Journal of Biochemistry , 111 :117 , 1980 |
| Abstract : Granon_1980_Eur.J.Biochem_111_117 |
| ESTHER : Granon_1980_Eur.J.Biochem_111_117 |
| PubMedSearch : Granon_1980_Eur.J.Biochem_111_117 |
| PubMedID: 7439178 |
| Gene_locus related to this paper: pig-1plip |