Triacetylfusigen
General
Type : Siderophore
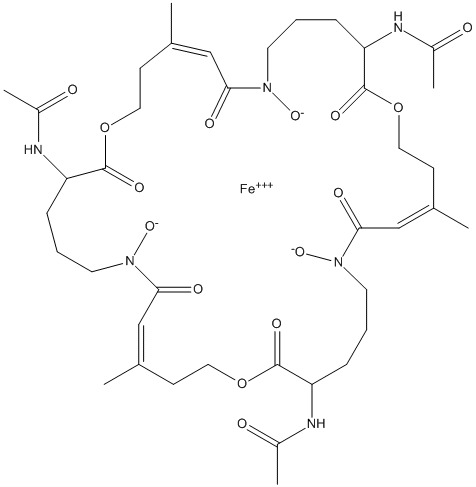
Chemical_Nomenclature : iron\;[(4Z,11S,16Z,23S,28Z,35S)-11,23,35-triacetamido-7,19,31-trihydroxy-4,16,28-trimethyl-12,24,36-trioxo-18,30-dioxoniumylidene-1,13,25-trioxa-7,19,31-triazacyclohexatriaconta-4,16,28-trien-6-ylidene]oxidanium
Canonical SMILES : CC1=CC(=[OH+])N(CCCC(C(=O)OCCC(=CC(=[OH+])N(CCCC(C(=O)OCCC(=CC(=[OH+])N(CCCC(C(=O)OCC1)NC(=O)C)O)C)NC(=O)C)O)C)NC(=O)C)O.[Fe]
InChI : InChI=1S\/C39H60N6O15.Fe\/c1-25-13-19-58-37(52)31(40-28(4)46)11-8-17-44(56)35(50)23-27(3)15-21-60-39(54)33(42-30(6)48)12-9-18-45(57)36(51)24-26(2)14-20-59-38(53)32(41-29(5)47)10-7-16-43(55)34(49)22-25\;\/h22-24,31-33,55-57H,7-21H2,1-6H3,(H,40,46)(H,41,47)(H,42,48)\;\/p+3\/b25-22-,26-24-,27-23-\;\/t31-,32-,33-\;\/m0.\/s1
InChIKey : PJECCKYLTMQOCP-IJGXQDLJSA-Q
Other name(s) : DF-TafC, desferri-triacetylfusarinine C, TAFSC, CHEBI:60481, Triacetylfusarinine C, Ferric triacetylfusarinine C, N,N,N-triacetylfusarinine C, N',N'',N'''-triacetylfusarinine C, {N,N',N''-[(3S,9Z,15S,21Z,27S,33Z)-7,19,31-tri(hydroxy-kappaO)-10,22,34-trimethyl-2,14,26-trioxo-8,20,32-tri(oxo-kappaO)-1,13,25-trioxa-7,19,31-triazacyclohexatriaconta-9,21,33-triene-3,15,27-triyl]triacetamidato(3-)}iron
Target
Families : A85-IroE-IroD-Fes-Yiel
References (5)
| Title : Iron Scavenging in Aspergillus Species: Structural and Biochemical Insights into Fungal Siderophore Esterases - Ecker_2018_Angew.Chem.Int.Ed.Engl_57_14624 |
| Author(s) : Ecker F , Haas H , Groll M , Huber EM |
| Ref : Angew Chem Int Ed Engl , 57 :14624 , 2018 |
| Abstract : Ecker_2018_Angew.Chem.Int.Ed.Engl_57_14624 |
| ESTHER : Ecker_2018_Angew.Chem.Int.Ed.Engl_57_14624 |
| PubMedSearch : Ecker_2018_Angew.Chem.Int.Ed.Engl_57_14624 |
| PubMedID: 30070018 |
| Gene_locus related to this paper: aspfu-q4wf29 , emeni-AnEstB , emeni-q5av79 , aspfu-q4wf56 |
| Title : An iron-mimicking, Trojan horse-entering fungi--has the time come for molecular imaging of fungal infections? - |
| Author(s) : Haas H , Petrik M , Decristoforo C |
| Ref : PLoS Pathog , 11 :e1004568 , 2015 |
| PubMedID: 25634225 |
| Title : Fungal siderophore metabolism with a focus on Aspergillus fumigatus - Haas_2014_Nat.Prod.Rep_31_1266 |
| Author(s) : Haas H |
| Ref : Nat Prod Rep , 31 :1266 , 2014 |
| Abstract : Haas_2014_Nat.Prod.Rep_31_1266 |
| ESTHER : Haas_2014_Nat.Prod.Rep_31_1266 |
| PubMedSearch : Haas_2014_Nat.Prod.Rep_31_1266 |
| PubMedID: 25140791 |
| Title : Optimization of triacetylfusarinine C and ferricrocin productions in Aspergillus fumigatus - Szigeti_2014_Acta.Microbiol.Immunol.Hung_61_107 |
| Author(s) : Szigeti ZM , Szaniszlo S , Fazekas E , Gyemant G , Szabon J , Antal K , Emri T , Balla J , Balla G , Csernoch L , Pocsi I |
| Ref : Acta Microbiol Immunol Hung , 61 :107 , 2014 |
| Abstract : Szigeti_2014_Acta.Microbiol.Immunol.Hung_61_107 |
| ESTHER : Szigeti_2014_Acta.Microbiol.Immunol.Hung_61_107 |
| PubMedSearch : Szigeti_2014_Acta.Microbiol.Immunol.Hung_61_107 |
| PubMedID: 24939680 |
| Title : EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus - Kragl_2007_Eukaryot.Cell_6_1278 |
| Author(s) : Kragl C , Schrettl M , Abt B , Sarg B , Lindner HH , Haas H |
| Ref : Eukaryot Cell , 6 :1278 , 2007 |
| Abstract : Kragl_2007_Eukaryot.Cell_6_1278 |
| ESTHER : Kragl_2007_Eukaryot.Cell_6_1278 |
| PubMedSearch : Kragl_2007_Eukaryot.Cell_6_1278 |
| PubMedID: 17586718 |
| Gene_locus related to this paper: aspfu-q4wf29 |