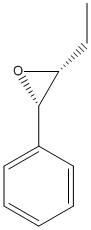
Trans-8-ethylstyrene-7,8-oxide
General
Type : Epoxide
Chemical_Nomenclature : (2R,3R)-2-ethyl-3-phenyloxirane
Canonical SMILES : CCC1C(O1)C2=CC=CC=C2
InChI : InChI=1S\/C10H12O\/c1-2-9-10(11-9)8-6-4-3-5-7-8\/h3-7,9-10H,2H2,1H3\/t9-,10-\/m1\/s1
InChIKey : BAFMBHJRYJBYQD-NXEZZACHSA-N
Other name(s) : TESO, trans-beta-Ethylstyrene 7,8-oxide, Oxirane, 2-ethyl-3-phenyl-, trans-, SCHEMBL7951294
Target
Families : Epoxide_hydrolase
References (5)
| Title : Identification and characterization of a new epoxide hydrolase from mouse liver microsomes - Guenthner_1983_J.Biol.Chem_258_15054 |
| Author(s) : Guenthner TM , Oesch F |
| Ref : Journal of Biological Chemistry , 258 :15054 , 1983 |
| Abstract : Guenthner_1983_J.Biol.Chem_258_15054 |
| ESTHER : Guenthner_1983_J.Biol.Chem_258_15054 |
| PubMedSearch : Guenthner_1983_J.Biol.Chem_258_15054 |
| PubMedID: 6654903 |
| Title : Microsomal and cytosolic epoxide hydrolases in rhesus monkey liver, and in normal and neoplastic human liver - Gill_1983_Life.Sci_32_2693 |
| Author(s) : Gill SS , Ota K , Ruebner B , Hammock BD |
| Ref : Life Sciences , 32 :2693 , 1983 |
| Abstract : Gill_1983_Life.Sci_32_2693 |
| ESTHER : Gill_1983_Life.Sci_32_2693 |
| PubMedSearch : Gill_1983_Life.Sci_32_2693 |
| PubMedID: 6855465 |
| Title : Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme - Wang_1982_Biochemistry_21_5769 |
| Author(s) : Wang P , Meijer J , Guengerich FP |
| Ref : Biochemistry , 21 :5769 , 1982 |
| Abstract : Wang_1982_Biochemistry_21_5769 |
| ESTHER : Wang_1982_Biochemistry_21_5769 |
| PubMedSearch : Wang_1982_Biochemistry_21_5769 |
| PubMedID: 6185139 |
| Gene_locus related to this paper: human-EPHX1 , human-EPHX2 |
| Title : Epoxide hydrolase activity in the mitochondrial fraction of mouse liver - Gill_1981_Nature_291_167 |
| Author(s) : Gill SS , Hammock BD |
| Ref : Nature , 291 :167 , 1981 |
| Abstract : Gill_1981_Nature_291_167 |
| ESTHER : Gill_1981_Nature_291_167 |
| PubMedSearch : Gill_1981_Nature_291_167 |
| PubMedID: 7194967 |
| Title : A rapid radiometric assay for mammalian cytosolic epoxide hydrolase - |
| Author(s) : Mullin CA , Hammock BD |
| Ref : Analytical Biochemistry , 106 :476 , 1980 |
| PubMedID: 7447013 |