Thifensulfuron-methyl
General
Type : Herbicide || Sulfonamide || Sulfur compound || Carboxamide || Urea derivative || Triazine
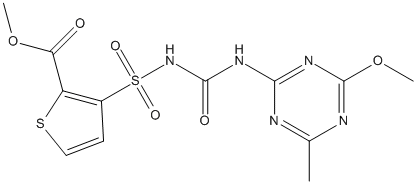
Chemical_Nomenclature : methyl 3-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoylsulfamoyl]thiophene-2-carboxylate
Canonical SMILES : CC1=NC(=NC(=N1)OC)NC(=O)NS(=O)(=O)C2=C(SC=C2)C(=O)OC
InChI : InChI=1S\/C12H13N5O6S2\/c1-6-13-10(16-12(14-6)23-3)15-11(19)17-25(20,21)7-4-5-24-8(7)9(18)22-2\/h4-5H,1-3H3,(H2,13,14,15,16,17,19)
InChIKey : AHTPATJNIAFOLR-UHFFFAOYSA-N
Other name(s) : Thifensulfuron methyl, Harmony, R4O, Pinnacle, SCHEMBL53606, CHEMBL1904815, SCHEMBL21300509, ZINC1532076
Target
Families : Bacterial_esterase
References (2)
| Title : Crystal structures of herbicide-detoxifying esterase reveal a lid loop affecting substrate binding and activity - Liu_2023_Nat.Commun_14_4343 |
| Author(s) : Liu B , Wang W , Qiu J , Huang X , Qiu S , Bao Y , Xu S , Ruan L , Ran T , He J |
| Ref : Nat Commun , 14 :4343 , 2023 |
| Abstract : Liu_2023_Nat.Commun_14_4343 |
| ESTHER : Liu_2023_Nat.Commun_14_4343 |
| PubMedSearch : Liu_2023_Nat.Commun_14_4343 |
| PubMedID: 37468532 |
| Gene_locus related to this paper: 9rhiz-g9i933 |
| Title : SulE, a sulfonylurea herbicide de-esterification esterase from Hansschlegelia zhihuaiae S113 - Hang_2012_Appl.Environ.Microbiol_78_1962 |
| Author(s) : Hang BJ , Hong Q , Xie XT , Huang X , Wang CH , He J , Li SP |
| Ref : Applied Environmental Microbiology , 78 :1962 , 2012 |
| Abstract : Hang_2012_Appl.Environ.Microbiol_78_1962 |
| ESTHER : Hang_2012_Appl.Environ.Microbiol_78_1962 |
| PubMedSearch : Hang_2012_Appl.Environ.Microbiol_78_1962 |
| PubMedID: 22247165 |
| Gene_locus related to this paper: 9rhiz-g9i933 |