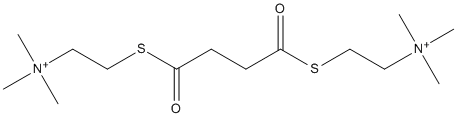
Succinyldithiocholine
General
Type : Trimethylammonium || Choline ester || Chromogen
Chemical_Nomenclature : trimethyl-[2-[4-oxo-4-[2-(trimethylazaniumyl)ethylsulfanyl]butanoyl]sulfanylethyl]azanium
Canonical SMILES : C[N+](C)(C)CCSC(=O)CCC(=O)SCC[N+](C)(C)C
InChI : InChI=1S\/C14H30N2O2S2\/c1-15(2,3)9-11-19-13(17)7-8-14(18)20-12-10-16(4,5)6\/h7-12H2,1-6H3\/q+2
InChIKey : UFPJLFHIAWIYIW-UHFFFAOYSA-N
Other name(s) : 2,2'-[(1,4-dioxobutane-1,4-diyl)disulfanediyl]bis(n,n,n-trimethylethanaminium
Target
Families : BCHE
References (3)
| Title : Effects of mutations of active site residues and amino acids interacting with the Omega loop on substrate activation of butyrylcholinesterase - Masson_2001_Biochim.Biophys.Acta_1544_166 |
| Author(s) : Masson P , Xie W , Froment MT , Lockridge O |
| Ref : Biochimica & Biophysica Acta , 1544 :166 , 2001 |
| Abstract : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| ESTHER : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedSearch : Masson_2001_Biochim.Biophys.Acta_1544_166 |
| PubMedID: 11341926 |
| Gene_locus related to this paper: human-BCHE |
| Title : Electroconvulsive therapy and the chronic use of pseudocholinesterase- inhibitor (echothiophate iodide) eye drops for glaucoma. A case report - Messer_1992_Gen.Hosp.Psychiatry_14_56 |
| Author(s) : Messer GJ , Stoudemire A , Knos G , Johnson GC |
| Ref : General Hospital Psychiatry , 14 :56 , 1992 |
| Abstract : Messer_1992_Gen.Hosp.Psychiatry_14_56 |
| ESTHER : Messer_1992_Gen.Hosp.Psychiatry_14_56 |
| PubMedSearch : Messer_1992_Gen.Hosp.Psychiatry_14_56 |
| PubMedID: 1730402 |
| Title : Kinetics of succinyldithiocholine hydrolysis by serum cholinesterase comparison to dibucaine and succinylcholine numbers - |
| Author(s) : Hersh LB , Raj PP , Ohlweiler D |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 189 :544 , 1974 |
| PubMedID: 4829230 |