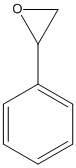
Styrene-oxide
General
Type : Epoxide
Chemical_Nomenclature : 2-phenyloxirane
Canonical SMILES : C1C(O1)C2=CC=CC=C2
InChI : InChI=1S\/C8H8O\/c1-2-4-7(5-3-1)8-6-9-8\/h1-5,8H,6H2
InChIKey : AWMVMTVKBNGEAK-MRVPVSSYSA-N
Other name(s) : Styrene oxide, 2-Phenyloxirane, Phenyloxirane, 1,2-Epoxyethylbenzene, Epoxystyrene, Phenylethylene oxide
MW : 120.14
Formula : C8H8O
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Epoxide_hydrolase, CFTR-inhibitory-factor_Cif
References (15)
| Title : Structural insights into the distinct substrate preferences of two bacterial epoxide hydrolases - Hwang_2024_Int.J.Biol.Macromol__130419 |
| Author(s) : Hwang J , Lee MJ , Lee SG , Do H , Lee JH |
| Ref : Int J Biol Macromol , :130419 , 2024 |
| Abstract : Hwang_2024_Int.J.Biol.Macromol__130419 |
| ESTHER : Hwang_2024_Int.J.Biol.Macromol__130419 |
| PubMedSearch : Hwang_2024_Int.J.Biol.Macromol__130419 |
| PubMedID: 38423431 |
| Gene_locus related to this paper: 9burk-a0a242m8j4 , 9hyph-a0a126p745 |
| Title : An epoxide hydrolase from endophytic Streptomyces shows unique structural features and wide biocatalytic activity - Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| Author(s) : Tormet-Gonzalez GD , Wilson C , de Oliveira GS , dos Santos JC , de Oliveira LG , Dias MVB |
| Ref : Acta Crystallographica D Struct Biol , 76 :868 , 2020 |
| Abstract : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| ESTHER : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| PubMedSearch : Tormet-Gonzalez_2020_Acta.Crystallogr.D.Struct.Biol_76_868 |
| PubMedID: 32876062 |
| Gene_locus related to this paper: strsq-b1eph2 |
| Title : Enantioselective Hydrolysis of Styrene Oxide and Benzyl Glycidyl Ether by a Variant of Epoxide Hydrolase from Agromyces mediolanus - Jin_2019_Mar.Drugs_17_ |
| Author(s) : Jin H , Li Y , Zhang Q , Lin S , Yang Z , Ding G |
| Ref : Mar Drugs , 17 : , 2019 |
| Abstract : Jin_2019_Mar.Drugs_17_ |
| ESTHER : Jin_2019_Mar.Drugs_17_ |
| PubMedSearch : Jin_2019_Mar.Drugs_17_ |
| PubMedID: 31226863 |
| Gene_locus related to this paper: agrme-a0a088b180 |
| Title : New Thermophilic alpha\/beta Class Epoxide Hydrolases Found in Metagenomes From Hot Environments - Ferrandi_2018_Front.Bioeng.Biotechnol_6_144 |
| Author(s) : Ferrandi EE , Sayer C , De Rose SA , Guazzelli E , Marchesi C , Saneei V , Isupov MN , Littlechild JA , Monti D |
| Ref : Front Bioeng Biotechnol , 6 :144 , 2018 |
| Abstract : Ferrandi_2018_Front.Bioeng.Biotechnol_6_144 |
| ESTHER : Ferrandi_2018_Front.Bioeng.Biotechnol_6_144 |
| PubMedSearch : Ferrandi_2018_Front.Bioeng.Biotechnol_6_144 |
| PubMedID: 30386778 |
| Gene_locus related to this paper: 9bact-a0a1u9wz52 , 9bact-a0a1u9wz58 |
| Title : Structural insights into human microsomal epoxide hydrolase by combined homology modeling, molecular dynamics simulations, and molecular docking calculations - Saenz-Mendez_2017_Proteins_85_720 |
| Author(s) : Saenz-Mendez P , Katz A , Perez-Kempner ML , Ventura ON , Vazquez M |
| Ref : Proteins , 85 :720 , 2017 |
| Abstract : Saenz-Mendez_2017_Proteins_85_720 |
| ESTHER : Saenz-Mendez_2017_Proteins_85_720 |
| PubMedSearch : Saenz-Mendez_2017_Proteins_85_720 |
| PubMedID: 28120429 |
| Title : mesT, a unique epoxide hydrolase, is essential for optimal growth of Mycobacterium tuberculosis in the presence of styrene oxide - Chownk_2017_Future.Microbiol_12_527 |
| Author(s) : Chownk M , Sharma A , Singh K , Kaur J |
| Ref : Future Microbiol , 12 :527 , 2017 |
| Abstract : Chownk_2017_Future.Microbiol_12_527 |
| ESTHER : Chownk_2017_Future.Microbiol_12_527 |
| PubMedSearch : Chownk_2017_Future.Microbiol_12_527 |
| PubMedID: 28492351 |
| Gene_locus related to this paper: myctu-LIPS |
| Title : Exploring the origins of selectivity in soluble epoxide hydrolase from Bacillus megaterium - Serrano-Hervas_2017_Org.Biomol.Chem_15_8827 |
| Author(s) : Serrano-Hervas E , Garcia-Borras M , Osuna S |
| Ref : Org Biomol Chem , 15 :8827 , 2017 |
| Abstract : Serrano-Hervas_2017_Org.Biomol.Chem_15_8827 |
| ESTHER : Serrano-Hervas_2017_Org.Biomol.Chem_15_8827 |
| PubMedSearch : Serrano-Hervas_2017_Org.Biomol.Chem_15_8827 |
| PubMedID: 29026902 |
| Title : Active-Site Flexibility and Substrate Specificity in a Bacterial Virulence Factor: Crystallographic Snapshots of an Epoxide Hydrolase - Hvorecny_2017_Structure_25_697 |
| Author(s) : Hvorecny KL , Bahl CD , Kitamura S , Lee KSS , Hammock BD , Morisseau C , Madden DR |
| Ref : Structure , 25 :697 , 2017 |
| Abstract : Hvorecny_2017_Structure_25_697 |
| ESTHER : Hvorecny_2017_Structure_25_697 |
| PubMedSearch : Hvorecny_2017_Structure_25_697 |
| PubMedID: 28392259 |
| Gene_locus related to this paper: pseae-PA2934 |
| Title : Kinetic resolution of racemic styrene oxide at a high concentration by recombinant Aspergillus usamii epoxide hydrolase in an n-hexanol\/buffer biphasic system - Hu_2016_J.Biotechnol_236_152 |
| Author(s) : Hu D , Wang R , Shi XL , Ye HH , Wu Q , Wu MC , Chu JJ |
| Ref : J Biotechnol , 236 :152 , 2016 |
| Abstract : Hu_2016_J.Biotechnol_236_152 |
| ESTHER : Hu_2016_J.Biotechnol_236_152 |
| PubMedSearch : Hu_2016_J.Biotechnol_236_152 |
| PubMedID: 27546798 |
| Gene_locus related to this paper: aspng-q1ktb5 |
| Title : Expression of a novel epoxide hydrolase of Aspergillus usamii E001 in Escherichia coli and its performance in resolution of racemic styrene oxide - Hu_2015_J.Ind.Microbiol.Biotechnol_42_671 |
| Author(s) : Hu D , Tang CD , Yang B , Liu JC , Yu T , Deng C , Wu MC |
| Ref : J Ind Microbiol Biotechnol , 42 :671 , 2015 |
| Abstract : Hu_2015_J.Ind.Microbiol.Biotechnol_42_671 |
| ESTHER : Hu_2015_J.Ind.Microbiol.Biotechnol_42_671 |
| PubMedSearch : Hu_2015_J.Ind.Microbiol.Biotechnol_42_671 |
| PubMedID: 25733186 |
| Gene_locus related to this paper: aspng-q1ktb5 |
| Title : Biochemical characterization and transcriptional analysis of the epoxide hydrolase from white-rot fungus Phanerochaete chrysosporium - Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| Author(s) : Li N , Zhang Y , Feng H |
| Ref : Acta Biochim Biophys Sin (Shanghai) , 41 :638 , 2009 |
| Abstract : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| ESTHER : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| PubMedSearch : Li_2009_Acta.Biochim.Biophys.Sin.(Shanghai)_41_638 |
| PubMedID: 19657565 |
| Gene_locus related to this paper: phach-b0fsq0 |
| Title : Enantioselective resolution of racemic styrene oxide at high concentration using recombinant Pichia pastoris expressing epoxide hydrolase of Rhodotorula glutinis in the presence of surfactant and glycerol - Yoo_2008_Biotechnol.Lett_30_1807 |
| Author(s) : Yoo SS , Park S , Lee EY |
| Ref : Biotechnol Lett , 30 :1807 , 2008 |
| Abstract : Yoo_2008_Biotechnol.Lett_30_1807 |
| ESTHER : Yoo_2008_Biotechnol.Lett_30_1807 |
| PubMedSearch : Yoo_2008_Biotechnol.Lett_30_1807 |
| PubMedID: 18575813 |
| Gene_locus related to this paper: rhogl-EPH1 |
| Title : A cold-adapted epoxide hydrolase from a strict marine bacterium, Sphingophyxis alaskensis - Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| Author(s) : Kang JH , Woo JH , Kang SG , Hwang YO , Kim SJ |
| Ref : J Microbiol Biotechnol , 18 :1445 , 2008 |
| Abstract : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| ESTHER : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedSearch : Kang_2008_J.Microbiol.Biotechnol_18_1445 |
| PubMedID: 18756107 |
| Gene_locus related to this paper: 9sphn-q3vax0 |
| Title : Protein engineering of epoxide hydrolase from Agrobacterium radiobacter AD1 for enhanced activity and enantioselective production of (R)-1-phenylethane-1,2-diol - Rui_2005_Appl.Environ.Microbiol_71_3995 |
| Author(s) : Rui L , Cao L , Chen W , Reardon KF , Wood TK |
| Ref : Applied Environmental Microbiology , 71 :3995 , 2005 |
| Abstract : Rui_2005_Appl.Environ.Microbiol_71_3995 |
| ESTHER : Rui_2005_Appl.Environ.Microbiol_71_3995 |
| PubMedSearch : Rui_2005_Appl.Environ.Microbiol_71_3995 |
| PubMedID: 16000814 |
| Gene_locus related to this paper: agrra-echA |
| Title : Directed evolution of epoxide hydrolase from A. radiobacter toward higher enantioselectivity by error-prone PCR and DNA shuffling - van Loo_2004_Chem.Biol_11_981 |
| Author(s) : van Loo B , Spelberg JH , Kingma J , Sonke T , Wubbolts MG , Janssen DB |
| Ref : Chemical Biology , 11 :981 , 2004 |
| Abstract : van Loo_2004_Chem.Biol_11_981 |
| ESTHER : van Loo_2004_Chem.Biol_11_981 |
| PubMedSearch : van Loo_2004_Chem.Biol_11_981 |
| PubMedID: 15271356 |
| Gene_locus related to this paper: agrra-echA |