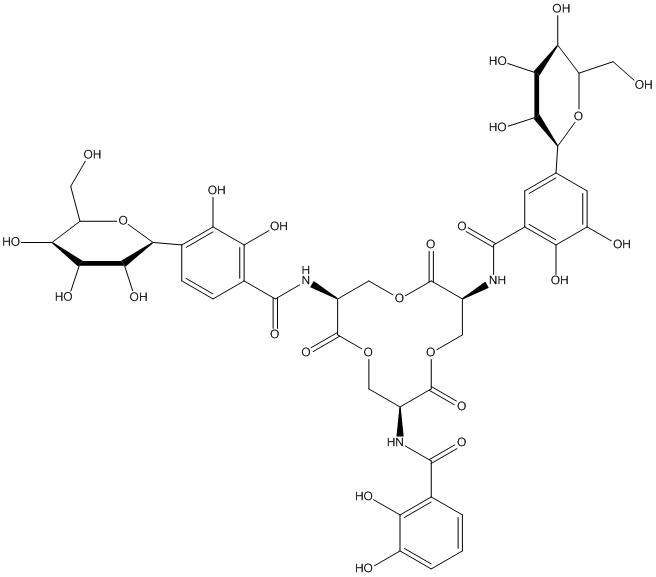
Salmochelin
General
Type : Siderophore || Carbohydrate
Chemical_Nomenclature : N-[(3S,7S,11S)-7-[(2,3-dihydroxybenzoyl)amino]-11-[[2,3-dihydroxy-5-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]benzoyl]amino]-2,6,10-trioxo-1,5,9-trioxacyclododec-3-yl]-2,3-dihydroxy-5-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]benzamide
Canonical SMILES : C1C(C(=O)OCC(C(=O)OCC(C(=O)O1)NC(=O)C2=CC(=CC(=C2O)O)C3C(C(C(C(O3)CO)O)O)O)NC(=O)C4=CC(=CC(=C4O)O)C5C(C(C(C(O5)CO)O)O)O)NC(=O)C6=C(C(=CC=C6)O)O
InChI : InChI=1S\/C42H47N3O25\/c46-8-24-29(54)31(56)33(58)35(69-24)13-4-16(27(52)22(49)6-13)38(61)44-19-11-67-40(63)18(43-37(60)15-2-1-3-21(48)26(15)51)10-66-41(64)20(12-68-42(19)65)45-39(62)17-5-14(7-23(50)28(17)53)36-34(59)32(57)30(55)25(9-47)70-36\/h1-7,18-20,24-25,29-36,46-59H,8-12H2,(H,43,60)(H,44,61)(H,45,62)\/t18-,19-,20-,24+,25+,29+,30+,31-,32-,33+,34+,35-,36-\/m0\/s1
InChIKey : NIGHGCIRXQBJIN-VUCKQUTDSA-N
Other name(s) : Salmochelin S4
Target
Families : A85-IroE-IroD-Fes-Yiel
References (1)
| Title : Structural characterization of enterobactin hydrolase IroE - Larsen_2006_Biochemistry_45_10184 |
| Author(s) : Larsen NA , Lin H , Wei R , Fischbach MA , Walsh CT |
| Ref : Biochemistry , 45 :10184 , 2006 |
| Abstract : Larsen_2006_Biochemistry_45_10184 |
| ESTHER : Larsen_2006_Biochemistry_45_10184 |
| PubMedSearch : Larsen_2006_Biochemistry_45_10184 |
| PubMedID: 16922493 |
| Gene_locus related to this paper: ecoli-IROE |