Sacubitril
General
Type : Pro-Drug || Not A\/B H target || Drug || Oxobutanoate
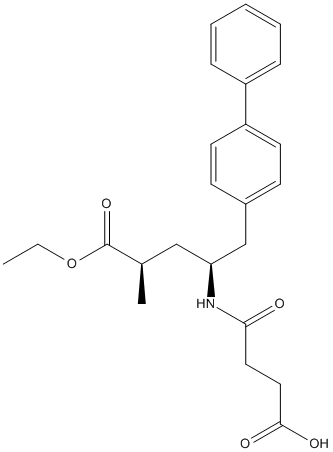
Chemical_Nomenclature : 4-[[(2S,4R)-5-ethoxy-4-methyl-5-oxo-1-(4-phenylphenyl)pentan-2-yl]amino]-4-oxobutanoic acid
Canonical SMILES : CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O)O
InChI : InChI=1S\/C24H29NO5\/c1-3-30-24(29)17(2)15-21(25-22(26)13-14-23(27)28)16-18-9-11-20(12-10-18)19-7-5-4-6-8-19\/h4-12,17,21H,3,13-16H2,1-2H3,(H,25,26)(H,27,28)\/t17-,21+\/m1\/s1
InChIKey : PYNXFZCZUAOOQC-UTKZUKDTSA-N
Other name(s) : AHU-377, AHU377, UNII-17ERJ0MKGI, AHU 377, LCZ 696, LCZ-696, Entresto
Target
Families : Carb_B_Chordata
References (3)
| Title : Transport properties of valsartan, sacubitril and its active metabolite (LBQ657) as determinants of disposition - Hanna_2017_Xenobiotica__1 |
| Author(s) : Hanna I , Alexander N , Crouthamel MH , Davis J , Natrillo A , Tran P , Vapurcuyan A , Zhu B |
| Ref : Xenobiotica , :1 , 2017 |
| Abstract : Hanna_2017_Xenobiotica__1 |
| ESTHER : Hanna_2017_Xenobiotica__1 |
| PubMedSearch : Hanna_2017_Xenobiotica__1 |
| PubMedID: 28281384 |
| Title : A Comprehensive Functional Assessment of Carboxylesterase 1 Nonsynonymous Polymorphisms - Wang_2017_Drug.Metab.Dispos_45_1149 |
| Author(s) : Wang X , Rida N , Shi J , Wu AH , Bleske BE , Zhu HJ |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 45 :1149 , 2017 |
| Abstract : Wang_2017_Drug.Metab.Dispos_45_1149 |
| ESTHER : Wang_2017_Drug.Metab.Dispos_45_1149 |
| PubMedSearch : Wang_2017_Drug.Metab.Dispos_45_1149 |
| PubMedID: 28838926 |
| Gene_locus related to this paper: human-CES1 |
| Title : Sacubitril Is Selectively Activated by Carboxylesterase 1 (CES1) in the Liver and the Activation Is Affected by CES1 Genetic Variation - Shi_2016_Drug.Metab.Dispos_44_554 |
| Author(s) : Shi J , Wang X , Nguyen J , Wu AH , Bleske BE , Zhu HJ |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 44 :554 , 2016 |
| Abstract : Shi_2016_Drug.Metab.Dispos_44_554 |
| ESTHER : Shi_2016_Drug.Metab.Dispos_44_554 |
| PubMedSearch : Shi_2016_Drug.Metab.Dispos_44_554 |
| PubMedID: 26817948 |
| Gene_locus related to this paper: human-CES1 |