Retinol-palmitate
General
Type : Natural || Retinol ester || Hexadecanoate || Palmitate
Chemical_Nomenclature : [(2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenyl] hexadecanoate
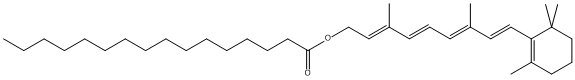
Canonical SMILES : CCCCCCCCCCCCCCCC(=O)OCC=C(C)C=CC=C(C)C=CC1=C(CCCC1(C)C)C
InChI : InChI=1S\/C36H60O2\/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-25-35(37)38-30-28-32(3)23-20-22-31(2)26-27-34-33(4)24-21-29-36(34,5)6\/h20,22-23,26-28H,7-19,21,24-25,29-30H2,1-6H3\/b23-20+,27-26+,31-22+,32-28+
InChIKey : VYGQUTWHTHXGQB-FFHKNEKCSA-N
Other name(s) : Vitamin A palmitate, retinyl palmitate, retinyl ester, Retinol palmitate, Retinol, hexadecanoate, CHEMBL1675, CHEBI:17616, SCHEMBL41649
Target
References (5)
| Title : Lysosomal acid lipase is the major acid retinyl ester hydrolase in cultured human hepatic stellate cells but not essential for retinyl ester degradation - Wagner_2020_Biochim.Biophys.Acta.Mol.Cell.Biol.Lipids_1865_158730 |
| Author(s) : Wagner C , Hois V , Pajed L , Pusch LM , Wolinski H , Trauner M , Zimmermann R , Taschler U , Lass A |
| Ref : Biochimica & Biophysica Acta Molecular & Cellular Biology Lipids , 1865 :158730 , 2020 |
| Abstract : Wagner_2020_Biochim.Biophys.Acta.Mol.Cell.Biol.Lipids_1865_158730 |
| ESTHER : Wagner_2020_Biochim.Biophys.Acta.Mol.Cell.Biol.Lipids_1865_158730 |
| PubMedSearch : Wagner_2020_Biochim.Biophys.Acta.Mol.Cell.Biol.Lipids_1865_158730 |
| PubMedID: 32361002 |
| Gene_locus related to this paper: human-LIPA |
| Title : Pancreatic lipase and pancreatic lipase-related protein 2, but not pancreatic lipase-related protein 1, hydrolyze retinyl palmitate in physiological conditions - Reboul_2006_Biochim.Biophys.Acta_1761_4 |
| Author(s) : Reboul E , Berton A , Moussa M , Kreuzer C , Crenon I , Borel P |
| Ref : Biochimica & Biophysica Acta , 1761 :4 , 2006 |
| Abstract : Reboul_2006_Biochim.Biophys.Acta_1761_4 |
| ESTHER : Reboul_2006_Biochim.Biophys.Acta_1761_4 |
| PubMedSearch : Reboul_2006_Biochim.Biophys.Acta_1761_4 |
| PubMedID: 16497549 |
| Gene_locus related to this paper: human-PNLIP , human-PNLIPRP2 |
| Title : Isolation and characterization of a microsomal acid retinyl ester hydrolase - Linke_2005_J.Biol.Chem_280_23287 |
| Author(s) : Linke T , Dawson H , Harrison EH |
| Ref : Journal of Biological Chemistry , 280 :23287 , 2005 |
| Abstract : Linke_2005_J.Biol.Chem_280_23287 |
| ESTHER : Linke_2005_J.Biol.Chem_280_23287 |
| PubMedSearch : Linke_2005_J.Biol.Chem_280_23287 |
| PubMedID: 15767260 |
| Gene_locus related to this paper: mouse-Ces1d , ratno-Ces1d |
| Title : Carotenol fatty acid esters: easy substrates for digestive enzymes? - Breithaupt_2002_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_132_721 |
| Author(s) : Breithaupt DE , Bamedi A , Wirt U |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 132 :721 , 2002 |
| Abstract : Breithaupt_2002_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_132_721 |
| ESTHER : Breithaupt_2002_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_132_721 |
| PubMedSearch : Breithaupt_2002_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_132_721 |
| PubMedID: 12128058 |
| Title : Hydrolysis of retinyl esters by non-specific carboxylesterases from rat liver endoplasmic reticulum - Mentlein_1987_Biochem.J_245_863 |
| Author(s) : Mentlein R , Heymann E |
| Ref : Biochemical Journal , 245 :863 , 1987 |
| Abstract : Mentlein_1987_Biochem.J_245_863 |
| ESTHER : Mentlein_1987_Biochem.J_245_863 |
| PubMedSearch : Mentlein_1987_Biochem.J_245_863 |
| PubMedID: 3663197 |