Remifentanil
General
Type : Drug || Not A\/B H target || Opioid analgesic
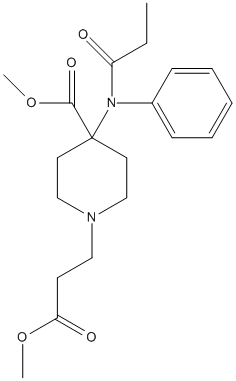
Chemical_Nomenclature : methyl 1-(3-methoxy-3-oxopropyl)-4-(N-propanoylanilino)piperidine-4-carboxylate
Canonical SMILES : CCC(=O)N(C1=CC=CC=C1)C2(CCN(CC2)CCC(=O)OC)C(=O)OC
InChI : InChI=1S\/C20H28N2O5\/c1-4-17(23)22(16-8-6-5-7-9-16)20(19(25)27-3)11-14-21(15-12-20)13-10-18(24)26-2\/h5-9H,4,10-15H2,1-3H3
InChIKey : ZTVQQQVZCWLTDF-UHFFFAOYSA-N
Other name(s) : Remifentanyl, Ultiva, Remifentanil [INN:BAN], UNII-P10582JYYK, Ultiva (TN)
MW : 376.44
Formula : C20H28N2O5
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carboxylesterase, Carb_B_Chordata
References (8)
| Title : Remifentanil: applications in neonates - Kamata_2016_J.Anesth_30_449 |
| Author(s) : Kamata M , Tobias JD |
| Ref : J Anesth , 30 :449 , 2016 |
| Abstract : Kamata_2016_J.Anesth_30_449 |
| ESTHER : Kamata_2016_J.Anesth_30_449 |
| PubMedSearch : Kamata_2016_J.Anesth_30_449 |
| PubMedID: 26758072 |
| Title : Customized anesthetic preservation of ictal threshold in electroconvulsive therapy: role of adjunctive remifentanil with etomidate - Augoustides_2005_J.ECT_21_128 |
| Author(s) : Augoustides JG , Hosalkar HH , O'Reardon JP , Kofke WA , Datto CJ |
| Ref : J Ect , 21 :128 , 2005 |
| Abstract : Augoustides_2005_J.ECT_21_128 |
| ESTHER : Augoustides_2005_J.ECT_21_128 |
| PubMedSearch : Augoustides_2005_J.ECT_21_128 |
| PubMedID: 15905758 |
| Title : Does pancuronium or cisatracurium delay the rate of arousal following remifentanil-based anesthesia? - Baraka_2005_Middle.East.J.Anaesthesiol_18_477 |
| Author(s) : Baraka AS , Haroun-Bizri ST , Nawfal MF , Gerges FJ , Nasr VG |
| Ref : Middle East J Anaesthesiol , 18 :477 , 2005 |
| Abstract : Baraka_2005_Middle.East.J.Anaesthesiol_18_477 |
| ESTHER : Baraka_2005_Middle.East.J.Anaesthesiol_18_477 |
| PubMedSearch : Baraka_2005_Middle.East.J.Anaesthesiol_18_477 |
| PubMedID: 16381256 |
| Title : In vitro remifentanil metabolism: the effects of whole blood constituents and plasma butyrylcholinesterase - Davis_2002_Anesth.Analg_95_1305 |
| Author(s) : Davis PJ , Stiller RL , Wilson AS , McGowan FX , Egan TD , Muir KT |
| Ref : Anesthesia & Analgesia , 95 :1305 , 2002 |
| Abstract : Davis_2002_Anesth.Analg_95_1305 |
| ESTHER : Davis_2002_Anesth.Analg_95_1305 |
| PubMedSearch : Davis_2002_Anesth.Analg_95_1305 |
| PubMedID: 12401616 |
| Title : The use of remifentanil for bloodless surgical field during vertebral disc resection - Chillemi_2002_Minerva.Anestesiol_68_645 |
| Author(s) : Chillemi S , Sinardi D , Marino A , Mantarro G , Campisi R |
| Ref : Minerva Anestesiol , 68 :645 , 2002 |
| Abstract : Chillemi_2002_Minerva.Anestesiol_68_645 |
| ESTHER : Chillemi_2002_Minerva.Anestesiol_68_645 |
| PubMedSearch : Chillemi_2002_Minerva.Anestesiol_68_645 |
| PubMedID: 12370680 |
| Title : Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal - Mortazavi_1999_Anesthesiology_90_1070 |
| Author(s) : Mortazavi S , Thompson J , Baghdoyan HA , Lydic R |
| Ref : Anesthesiology , 90 :1070 , 1999 |
| Abstract : Mortazavi_1999_Anesthesiology_90_1070 |
| ESTHER : Mortazavi_1999_Anesthesiology_90_1070 |
| PubMedSearch : Mortazavi_1999_Anesthesiology_90_1070 |
| PubMedID: 10201679 |
| Title : [Esters and stereoisomers] - Nigrovic_1997_Anaesthesist_46_282 |
| Author(s) : Nigrovic V , Diefenbach C , Mellinghoff H |
| Ref : Anaesthesist , 46 :282 , 1997 |
| Abstract : Nigrovic_1997_Anaesthesist_46_282 |
| ESTHER : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedSearch : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedID: 9229981 |