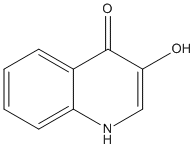
Quinoline-3,4-diol
General
Type : Quinoline
Chemical_Nomenclature : 3-hydroxy-1H-quinolin-4-one
Canonical SMILES : C1=CC=C2C(=C1)C(=O)C(=CN2)O
InChI : InChI=1S\/C9H7NO2\/c11-8-5-10-7-4-2-1-3-6(7)9(8)12\/h1-5,11H,(H,10,12)
InChIKey : BHTNYVRPYQQOMJ-UHFFFAOYSA-N
Other name(s) : HQ, 3-hydroxy-4(1H)-quinolone, 1-H-3-hydroxy-4-oxoquinoline, 3,4-Dihydroxyquinoline, 3-Hydroxy-1H-quinolin-4-one, QNL, 3-hydroxyquinolin-4(1H)-one
Target
Families : HOD-cofactorfree-dioxygenase
References (8)
| Title : Enzyme-Mediated Quenching of the Pseudomonas Quinolone Signal (PQS): A Comparison between Naturally Occurring and Engineered PQS-Cleaving Dioxygenases - Arranz San Martin_2022_Biomolecules_12_170 |
| Author(s) : Arranz San Martin A , Vogel J , Wullich SC , Quax WJ , Fetzner S |
| Ref : Biomolecules , 12 :170 , 2021 |
| Abstract : Arranz San Martin_2022_Biomolecules_12_170 |
| ESTHER : Arranz San Martin_2022_Biomolecules_12_170 |
| PubMedSearch : Arranz San Martin_2022_Biomolecules_12_170 |
| PubMedID: |
| Gene_locus related to this paper: artsp-hod , strbb-d7bw96 , mycab-x8en65 , nocfa-q5yp20 |
| Title : Bio-inspired flavonol and quinolone dioxygenation by a non-heme iron catalyst modeling the action of flavonol and 3-hydroxy-4(1H)-quinolone 2,4-dioxygenases - Pap_2012_J.Inorg.Biochem_108_15 |
| Author(s) : Pap JS , Matuz A , Barath G , Kripli B , Giorgi M , Speier G , Kaizer J |
| Ref : J Inorg Biochem , 108 :15 , 2012 |
| Abstract : Pap_2012_J.Inorg.Biochem_108_15 |
| ESTHER : Pap_2012_J.Inorg.Biochem_108_15 |
| PubMedSearch : Pap_2012_J.Inorg.Biochem_108_15 |
| PubMedID: 22265834 |
| Title : Quorum quenching revisited--from signal decays to signalling confusion - Hong_2012_Sensors.(Basel)_12_4661 |
| Author(s) : Hong KW , Koh CL , Sam CK , Yin WF , Chan KG |
| Ref : Sensors (Basel) , 12 :4661 , 2012 |
| Abstract : Hong_2012_Sensors.(Basel)_12_4661 |
| ESTHER : Hong_2012_Sensors.(Basel)_12_4661 |
| PubMedSearch : Hong_2012_Sensors.(Basel)_12_4661 |
| PubMedID: 22666051 |
| Title : Structural basis for cofactor-independent dioxygenation of N-heteroaromatic compounds at the alpha\/beta-hydrolase fold - Steiner_2010_Proc.Natl.Acad.Sci.U.S.A_107_657 |
| Author(s) : Steiner RA , Janssen HJ , Roversi P , Oakley AJ , Fetzner S |
| Ref : Proc Natl Acad Sci U S A , 107 :657 , 2010 |
| Abstract : Steiner_2010_Proc.Natl.Acad.Sci.U.S.A_107_657 |
| ESTHER : Steiner_2010_Proc.Natl.Acad.Sci.U.S.A_107_657 |
| PubMedSearch : Steiner_2010_Proc.Natl.Acad.Sci.U.S.A_107_657 |
| PubMedID: 20080731 |
| Gene_locus related to this paper: artsp-hod , psepu-QDO |
| Title : Cofactor-independent oxidases and oxygenases - Fetzner_2010_Appl.Microbiol.Biotechnol_86_791 |
| Author(s) : Fetzner S , Steiner RA |
| Ref : Applied Microbiology & Biotechnology , 86 :791 , 2010 |
| Abstract : Fetzner_2010_Appl.Microbiol.Biotechnol_86_791 |
| ESTHER : Fetzner_2010_Appl.Microbiol.Biotechnol_86_791 |
| PubMedSearch : Fetzner_2010_Appl.Microbiol.Biotechnol_86_791 |
| PubMedID: 20157809 |
| Title : Crystallization and diffraction data of 1H-3-hydroxy-4-oxoquinoline 2,4-dioxygenase: a cofactor-free oxygenase of the alpha\/beta-hydrolase family - Qi_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_378 |
| Author(s) : Qi R , Fetzner S , Oakley AJ |
| Ref : Acta Crystallographica Sect F Struct Biol Cryst Commun , 63 :378 , 2007 |
| Abstract : Qi_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_378 |
| ESTHER : Qi_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_378 |
| PubMedSearch : Qi_2007_Acta.Crystallogr.Sect.F.Struct.Biol.Cryst.Commun_63_378 |
| PubMedID: 17565175 |
| Title : Dioxygenases without requirement for cofactors and their chemical model reaction: compulsory order ternary complex mechanism of 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase involving general base catalysis by histidine 251 and single-electron oxidation of the substrate dianion - Frerichs-Deeken_2004_Biochemistry_43_14485 |
| Author(s) : Frerichs-Deeken U , Ranguelova K , Kappl R , Huttermann J , Fetzner S |
| Ref : Biochemistry , 43 :14485 , 2004 |
| Abstract : Frerichs-Deeken_2004_Biochemistry_43_14485 |
| ESTHER : Frerichs-Deeken_2004_Biochemistry_43_14485 |
| PubMedSearch : Frerichs-Deeken_2004_Biochemistry_43_14485 |
| PubMedID: 15533053 |
| Gene_locus related to this paper: artsp-hod |
| Title : 2,4-dioxygenases catalyzing N-heterocyclic-ring cleavage and formation of carbon monoxide. Purification and some properties of 1H-3-hydroxy-4-oxoquinaldine 2,4-dioxygenase from Arthrobacter sp. Ru61a and comparison with 1H-3-hydroxy-4-oxoquinoline 2,4-dioxygenase from Pseudomonas putida 33\/1 - Bauer_1996_Eur.J.Biochem_240_576 |
| Author(s) : Bauer I , Max N , Fetzner S , Lingens F |
| Ref : European Journal of Biochemistry , 240 :576 , 1996 |
| Abstract : Bauer_1996_Eur.J.Biochem_240_576 |
| ESTHER : Bauer_1996_Eur.J.Biochem_240_576 |
| PubMedSearch : Bauer_1996_Eur.J.Biochem_240_576 |
| PubMedID: 8856057 |
| Gene_locus related to this paper: artsp-hod , psepu-QDO |