QMiPSB
General
Type : Cannabinoid-Receptor-ligand || Sulfur compound || Sulfonamide || Quinoline
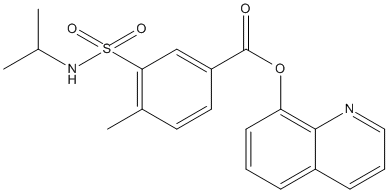
Chemical_Nomenclature : quinolin-8-yl 4-methyl-3-[(propan-2-yl)sulfamoyl]benzoate
Canonical SMILES : [C]1=[C][C]=C3C(=C1OC(=O)C2=[C]C(=C([C]=[C]2)[C])[S]([N][C]([C])[C])(=O)=O)N=[C][C]=[C]3
InChI : InChI=1S\/C20H20N2O4S\/c1-13(2)22-27(24,25)18-12-16(10-9-14(18)3)20(23)26-17-8-4-6-15-7-5-11-21-19(15)17\/h4-13,22H,1-3H3
InChIKey : PFPYPILLHUNDMZ-UHFFFAOYSA-N
Other name(s) : SGT-46
MW : 384.44
Formula : C20H20N2O4S
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carb_B_Chordata
References (2)
| Title : In vitro metabolic fate of the synthetic cannabinoid receptor agonists QMMSB (quinolin-8-yl 4-methyl-3-(morpholine-4-sulfonyl)benzoate) and QMiPSB (quinolin-8-yl 4-methyl-3-[(propan-2-yl)sulfamoyl]benzoate) including isozyme mapping and carboxylesterases activity testing - Richter_2022_Drug.Test.Anal__ |
| Author(s) : Richter MJ , Wagmann L , Kavanagh PV , Brandt SD , Meyer MR |
| Ref : Drug Test Anal , : , 2022 |
| Abstract : Richter_2022_Drug.Test.Anal__ |
| ESTHER : Richter_2022_Drug.Test.Anal__ |
| PubMedSearch : Richter_2022_Drug.Test.Anal__ |
| PubMedID: 36239626 |
| Title : Synthetic cannabinoid receptor agonists: Analytical profiles and development of QMPSB, QMMSB, QMPCB, 2F-QMPSB, QMiPSB, and SGT-233 - Brandt_2021_Drug.Test.Anal_13_175 |
| Author(s) : Brandt SD , Kavanagh PV , Westphal F , Dreiseitel W , Dowling G , Bowden MJ , Williamson JPB |
| Ref : Drug Test Anal , 13 :175 , 2021 |
| Abstract : Brandt_2021_Drug.Test.Anal_13_175 |
| ESTHER : Brandt_2021_Drug.Test.Anal_13_175 |
| PubMedSearch : Brandt_2021_Drug.Test.Anal_13_175 |
| PubMedID: 32880103 |