PyiE-Substrate
General
Type : Mycotoxin-metabolite || Natural || Toxin
Chemical_Nomenclature :
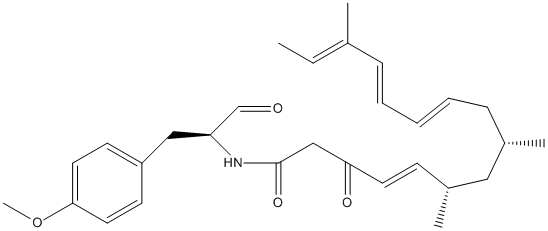
Canonical SMILES : C(=CC[C@@H](C[C@@H](C=CC(=O)CC(=O)N[C@H](C=O)CC1=CC=C(C=C1)OC)C)C)C=CC(=CC)C
InChI : InChI=1S\/C29H39NO4\/c1-6-22(2)10-8-7-9-11-23(3)18-24(4)12-15-27(32)20-29(33)30-26(21-31)19-25-13-16-28(34-5)17-14-25\/h6-10,12-17,21,23-24,26H,11,18-20H2,1-5H3,(H,30,33)\/t23-,24+,26-\/m0\/s1
InChIKey : MZCYDAXULWAETG-GSLIJJQTSA-N
Other name(s) :
MW : 465.63
Formula : C29H39NO4
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Duf_1100-S
References (1)
| Title : Chemical and Genetic Studies on the Formation of Pyrrolones During the Biosynthesis of Cytochalasans - Zhang_2021_Chemistry_10_3106 |
| Author(s) : Zhang H , Hantke V , Bruhnke P , Skellam E , Cox RJ |
| Ref : Chemistry , 10 :3106 , 2021 |
| Abstract : Zhang_2021_Chemistry_10_3106 |
| ESTHER : Zhang_2021_Chemistry_10_3106 |
| PubMedSearch : Zhang_2021_Chemistry_10_3106 |
| PubMedID: 33146923 |
| Gene_locus related to this paper: phano-phmG , aspcl-CCSE , aspfu-psoB , mago7-ORFZB , maggr-pyie |