Prostaglandin-D2-Glyceryl-Ester
General
Type : Prostaglandin || Acyl-Glycerol || Cannabinoid-Receptor-ligand || Natural
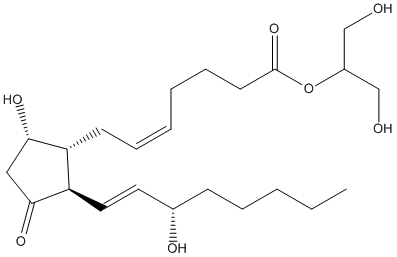
Chemical_Nomenclature : 1,3-dihydroxypropan-2-yl (Z)-7-[(1R,2R,5S)-5-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-3-oxocyclopentyl]hept-5-enoate
Canonical SMILES : CCCCCC(C=CC1C(C(CC1=O)O)CC=CCCCC(=O)OC(CO)CO)O
InChI : InChI=1S\/C23H38O7\/c1-2-3-6-9-17(26)12-13-20-19(21(27)14-22(20)28)10-7-4-5-8-11-23(29)30-18(15-24)16-25\/h4,7,12-13,17-21,24-27H,2-3,5-6,8-11,14-16H2,1H3\/b7-4-,13-12+\/t17-,19+,20+,21-\/m0\/s1
InChIKey : OCYWGBZEVKVWBQ-PQGWWSFGSA-N
Other name(s) : Prostaglandin D(2) Glyceryl Ester, 2-Glyceryl-Prostaglandin D2, 2-glyceryl-PGD2, 9S,15S-dihydroxy-11-oxo-5Z,13E-prostadienoic acid 2-glyceryl ester, CHEBI:133979, prostaglandin D2 2-glyceryl ester
MW : 426.5
Formula : C23H38O7
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Carb_B_Chordata
References (2)
| Title : Inactivation of CES1 Blocks Prostaglandin D(2) Glyceryl Ester Catabolism in Monocytes\/Macrophages and Enhances Its Anti-inflammatory Effects, Whereas the Pro-inflammatory Effects of Prostaglandin E(2) Glyceryl Ester Are Attenuated - Scheaffer_2020_ACS.Omega_5_29177 |
| Author(s) : Scheaffer HL , Borazjani A , Szafran BN , Ross MK |
| Ref : ACS Omega , 5 :29177 , 2020 |
| Abstract : Scheaffer_2020_ACS.Omega_5_29177 |
| ESTHER : Scheaffer_2020_ACS.Omega_5_29177 |
| PubMedSearch : Scheaffer_2020_ACS.Omega_5_29177 |
| PubMedID: 33225149 |
| Gene_locus related to this paper: human-CES1 |
| Title : Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides - Kozak_2002_J.Biol.Chem_277_44877 |
| Author(s) : Kozak KR , Crews BC , Morrow JD , Wang LH , Ma YH , Weinander R , Jakobsson PJ , Marnett LJ |
| Ref : Journal of Biological Chemistry , 277 :44877 , 2002 |
| Abstract : Kozak_2002_J.Biol.Chem_277_44877 |
| ESTHER : Kozak_2002_J.Biol.Chem_277_44877 |
| PubMedSearch : Kozak_2002_J.Biol.Chem_277_44877 |
| PubMedID: 12244105 |