Propyl-gallate
General
Type : Derivative of Gallate || Natural || Polyphenol || Benzoate
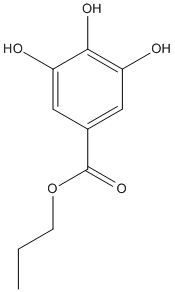
Chemical_Nomenclature : propyl 3,4,5-trihydroxybenzoate
Canonical SMILES : CCCOC(=O)C1=CC(=C(C(=C1)O)O)O
InChI : InChI=1S\/C10H12O5\/c1-2-3-15-10(14)6-4-7(11)9(13)8(12)5-6\/h4-5,11-13H,2-3H2,1H3
InChIKey : ZTHYODDOHIVTJV-UHFFFAOYSA-N
Other name(s) : propyl gallate, Propyl 3,4,5-trihydroxybenzoate, N-Propyl gallate, Gallic acid, propyl ester
Target
Families : Plant_carboxylesterase
References (4)
| Title : Discovery and characterization of tannase genes in plants: roles in hydrolysis of tannins - Dai_2020_New.Phytol_226_1104 |
| Author(s) : Dai X , Liu Y , Zhuang J , Yao S , Liu L , Jiang X , Zhou K , Wang Y , Xie D , Bennetzen JL , Gao L , Xia T |
| Ref : New Phytol , 226 :1104 , 2020 |
| Abstract : Dai_2020_New.Phytol_226_1104 |
| ESTHER : Dai_2020_New.Phytol_226_1104 |
| PubMedSearch : Dai_2020_New.Phytol_226_1104 |
| PubMedID: 32061142 |
| Gene_locus related to this paper: camsi-a0a5b9g8c2 , jugre-JrTA , fraan-FaTA , vitvi-VvTA , citcl-CcTA , dioka-DkTA , camsi-CsTA |
| Title : Propyl gallate synthesis using acidophilic tannase and simultaneous production of tannase and gallic acid by marine Aspergillus awamori BTMFW032 - Beena_2011_Appl.Biochem.Biotechnol_164_612 |
| Author(s) : Beena PS , Basheer SM , Bhat SG , Bahkali AH , Chandrasekaran M |
| Ref : Appl Biochem Biotechnol , 164 :612 , 2011 |
| Abstract : Beena_2011_Appl.Biochem.Biotechnol_164_612 |
| ESTHER : Beena_2011_Appl.Biochem.Biotechnol_164_612 |
| PubMedSearch : Beena_2011_Appl.Biochem.Biotechnol_164_612 |
| PubMedID: 21279470 |
| Title : Synthesis of propyl gallate by transesterification of tannic acid in aqueous media catalysed by immobilised derivatives of tannase from Lactobacillus plantarum - Fernandez-Lorente_2011_Food.Chem_128_214 |
| Author(s) : Fernandez-Lorente G , Bolivar JM , Rocha-Martin J , Curiel JA , Munoz R , de Las Rivas B , Carrascosa AV , Guisan JM |
| Ref : Food Chem , 128 :214 , 2011 |
| Abstract : Fernandez-Lorente_2011_Food.Chem_128_214 |
| ESTHER : Fernandez-Lorente_2011_Food.Chem_128_214 |
| PubMedSearch : Fernandez-Lorente_2011_Food.Chem_128_214 |
| PubMedID: 25214351 |
| Title : Ecotoxicological effects of the antioxidant additive propyl gallate in five aquatic systems - Zurita_2007_Water.Res_41_2599 |
| Author(s) : Zurita JL , Jos A , del Peso A , Salguero M , Lopez-Artiguez M , Repetto G |
| Ref : Water Res , 41 :2599 , 2007 |
| Abstract : Zurita_2007_Water.Res_41_2599 |
| ESTHER : Zurita_2007_Water.Res_41_2599 |
| PubMedSearch : Zurita_2007_Water.Res_41_2599 |
| PubMedID: 17382989 |