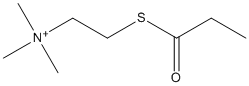
Propionylthiocholine
General
Type : Propionate || Trimethylammonium || Choline ester || Chromogen
Chemical_Nomenclature : trimethyl(2-propanoylsulfanylethyl)azanium
Canonical SMILES : CCC(=O)SCC[N+](C)(C)C
InChI : InChI=1S\/C8H18NOS\/c1-5-8(10)11-7-6-9(2,3)4\/h5-7H2,1-4H3\/q+1
InChIKey : ILWCNQXVCCMVCY-UHFFFAOYSA-N
Other name(s) : Ethanaminium, N,N,N-trimethyl-2-((1-oxopropyl)thio)-, AC1L2MBR, AC1Q68WI, SCHEMBL1057055
References (4)
| Title : An explanation for the different inhibitory characteristics of human serum butyrylcholinesterase phenotypes deriving from inhibition of atypical heterozygotes - Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_159 |
| Author(s) : Simeon-Rudolf V , Kovarik Z , Skrinjaric-Spoljar M , Evans RT |
| Ref : Chemico-Biological Interactions , 119-120 :159 , 1999 |
| Abstract : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_159 |
| ESTHER : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_159 |
| PubMedSearch : Simeon-Rudolf_1999_Chem.Biol.Interact_119-120_159 |
| PubMedID: 10421449 |
| Title : [Comparative characterization of the catalytic properties of blood serum cholinesterase in pigeon and hen]. [Russian] - Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| Author(s) : Zhukovskii Iu G , Kuznestova LP , Sochilina EE , Fartseiger AG , Fartseiger NL , Azliarova MA |
| Ref : Ukrainskii Biokhimicheskii Zhurnal , 66 :23 , 1994 |
| Abstract : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| ESTHER : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| PubMedSearch : Zhukovskii_1994_Ukrainskii.Biokhimicheskii.Zhurnal_66_23 |
| PubMedID: 7747340 |
| Title : Polymorphism of pseudocholinesterase in Torpedo marmorata tissues: comparative study of the catalytic and molecular properties of this enzyme with acetylcholinesterase - Toutant_1985_J.Neurochem_44_580 |
| Author(s) : Toutant JP , Massoulie J , Bon S |
| Ref : Journal of Neurochemistry , 44 :580 , 1985 |
| Abstract : Toutant_1985_J.Neurochem_44_580 |
| ESTHER : Toutant_1985_J.Neurochem_44_580 |
| PubMedSearch : Toutant_1985_J.Neurochem_44_580 |
| PubMedID: 2578181 |
| Title : Comparison of a commercially available assay system with two reference methods for the determination of plasma cholinesterase variants - Whittaker_1983_Clin.Chem_29_1746 |
| Author(s) : Whittaker M , Britten JJ , Dawson PJ |
| Ref : Clinical Chemistry , 29 :1746 , 1983 |
| Abstract : Whittaker_1983_Clin.Chem_29_1746 |
| ESTHER : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedSearch : Whittaker_1983_Clin.Chem_29_1746 |
| PubMedID: 6616819 |