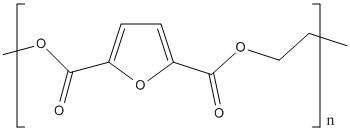
Polyethylene-2,5-furandicarboxylate
General
Type : Polymer || Polyester
Chemical_Nomenclature :
Canonical SMILES :
InChI :
InChIKey :
Other name(s) : Polyethylene 2,5-furandicarboxylate, PEF, Polyethylene furanoate, Polyethylene furandicarboxylate, Poly(ethylene furanoate)
MW :
Formula :
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Polyesterase-lipase-cutinase
References (4)
| Title : Efficient depolymerization of polyethylene terephthalate (PET) and polyethylene furanoate by engineered PET hydrolase Cut190 - Kawai_2022_AMB.Express_12_134 |
| Author(s) : Kawai F , Furushima Y , Mochizuki N , Muraki N , Yamashita M , Iida A , Mamoto R , Tosha T , Iizuka R , Kitajima S |
| Ref : AMB Express , 12 :134 , 2022 |
| Abstract : Kawai_2022_AMB.Express_12_134 |
| ESTHER : Kawai_2022_AMB.Express_12_134 |
| PubMedSearch : Kawai_2022_AMB.Express_12_134 |
| PubMedID: 36289098 |
| Gene_locus related to this paper: sacvd-c7mve8 |
| Title : Characterization and engineering of a plastic-degrading aromatic polyesterase - Austin_2018_Proc.Natl.Acad.Sci.U.S.A_115_E4350 |
| Author(s) : Austin HP , Allen MD , Donohoe BS , Rorrer NA , Kearns FL , Silveira RL , Pollard BC , Dominick G , Duman R , El Omari K , Mykhaylyk V , Wagner A , Michener WE , Amore A , Skaf MS , Crowley MF , Thorne AW , Johnson CW , Woodcock HL , McGeehan JE , Beckham GT |
| Ref : Proc Natl Acad Sci U S A , 115 :E4350 , 2018 |
| Abstract : Austin_2018_Proc.Natl.Acad.Sci.U.S.A_115_E4350 |
| ESTHER : Austin_2018_Proc.Natl.Acad.Sci.U.S.A_115_E4350 |
| PubMedSearch : Austin_2018_Proc.Natl.Acad.Sci.U.S.A_115_E4350 |
| PubMedID: 29666242 |
| Gene_locus related to this paper: idesa-peth |
| Title : Enzymatic hydrolysis of poly(ethylene furanoate) - Pellis_2016_J.Biotechnol_235_47 |
| Author(s) : Pellis A , Haernvall K , Pichler CM , Ghazaryan G , Breinbauer R , Guebitz GM |
| Ref : J Biotechnol , 235 :47 , 2016 |
| Abstract : Pellis_2016_J.Biotechnol_235_47 |
| ESTHER : Pellis_2016_J.Biotechnol_235_47 |
| PubMedSearch : Pellis_2016_J.Biotechnol_235_47 |
| PubMedID: 26854948 |
| Gene_locus related to this paper: thefu-q6a0i4 |
| Title : Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art - Sheldon_2014_Green.Chem_16_950 |
| Author(s) : Sheldon RA |
| Ref : Green Chem , 16 :950 , 2014 |
| Abstract : Sheldon_2014_Green.Chem_16_950 |
| ESTHER : Sheldon_2014_Green.Chem_16_950 |
| PubMedSearch : Sheldon_2014_Green.Chem_16_950 |
| PubMedID: |