Pimeloyl-ACP-methyl-ester
General
Type : Acyl peptide
Chemical_Nomenclature : Pimeloyl-ACP-methyl-ester
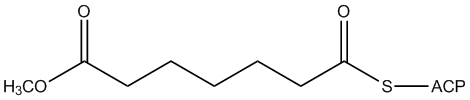
Canonical SMILES :
InChI :
InChIKey :
Other name(s) :
MW :
Formula : C7H12O3S-ACP
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
References (3)
| Title : A Francisella virulence factor catalyses an essential reaction of biotin synthesis - Feng_2014_Mol.Microbiol_91_300 |
| Author(s) : Feng Y , Napier BA , Manandhar M , Henke SK , Weiss DS , Cronan JE |
| Ref : Molecular Microbiology , 91 :300 , 2014 |
| Abstract : Feng_2014_Mol.Microbiol_91_300 |
| ESTHER : Feng_2014_Mol.Microbiol_91_300 |
| PubMedSearch : Feng_2014_Mol.Microbiol_91_300 |
| PubMedID: 24313380 |
| Gene_locus related to this paper: 9gamm-BioJ , fratt-q5nga8 |
| Title : Evolution of a new function in an esterase: simple amino acid substitutions enable the activity present in the larger paralog, BioH - Flores_2012_Protein.Eng.Des.Sel_25_387 |
| Author(s) : Flores H , Lin S , Contreras-Ferrat G , Cronan JE , Morett E |
| Ref : Protein Engineering Des Sel , 25 :387 , 2012 |
| Abstract : Flores_2012_Protein.Eng.Des.Sel_25_387 |
| ESTHER : Flores_2012_Protein.Eng.Des.Sel_25_387 |
| PubMedSearch : Flores_2012_Protein.Eng.Des.Sel_25_387 |
| PubMedID: 22691705 |
| Gene_locus related to this paper: pseae-PA3859 |
| Title : Biotin synthesis begins by hijacking the fatty acid synthetic pathway. - Lin_2010_Nat.Chem.Biol_6_682 |
| Author(s) : Lin S , Hanson RE , Cronan JE |
| Ref : Nat Chemical Biology , 6 :682 , 2010 |
| Abstract : Lin_2010_Nat.Chem.Biol_6_682 |
| ESTHER : Lin_2010_Nat.Chem.Biol_6_682 |
| PubMedSearch : Lin_2010_Nat.Chem.Biol_6_682 |
| PubMedID: 20693992 |
| Gene_locus related to this paper: 9gamm-BioJ , ecoli-bioh |