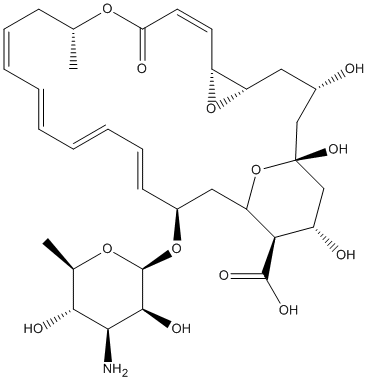
Pimaricin
General
Type : Natural || Macrolide || Antibiotic
Chemical_Nomenclature : (1R,3S,5R,7R,8E,12R,14E,16E,18E,20E,22R,24S,25R,26S)-22-[(2R,3S,4S,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-1,3,26-trihydroxy-12-methyl-10-oxo-6,11,28-trioxatricyclo[22.3.1.05,7]octacosa-8,14,16,18,20-pentaene-25-carboxylic acid
Canonical SMILES : CC1CC=CC=CC=CC=CC(CC2C(C(CC(O2)(CC(CC3C(O3)C=CC(=O)O1)O)O)O)C(=O)O)OC4C(C(C(C(O4)C)O)N)O
InChI : InChI=1S\/C33H47NO13\/c1-18-10-8-6-4-3-5-7-9-11-21(45-32-30(39)28(34)29(38)19(2)44-32)15-25-27(31(40)41)22(36)17-33(42,47-25)16-20(35)14-24-23(46-24)12-13-26(37)43-18\/h3-9,11-13,18-25,27-30,32,35-36,38-39,42H,10,14-17,34H2,1-2H3,(H,40,41)\/b4-3+,7-5+,8-6+,11-9+,13-12+\/t18-,19-,20+,21+,22+,23-,24-,25+,27-,28+,29-,30+,32+,33-\/m1\/s1
InChIKey : NCXMLFZGDNKEPB-FFPOYIOWSA-N
Other name(s) : Natamycin, Mycophyt, Synogil, Tennecetin, Delvocid
Target
Families : Thioesterase
References (2)
| Title : Structural and Mechanistic Insights into Chain Release of the Polyene PKS Thioesterase Domain - Zhou_2022_ACS.Catal_12_762 |
| Author(s) : Zhou Y , Tao W , Qi Z , Wei J , Shi T , Kang Q , Zheng J , Zhao Y , Bai L |
| Ref : ACS Catal , 12 :762 , 2022 |
| Abstract : Zhou_2022_ACS.Catal_12_762 |
| ESTHER : Zhou_2022_ACS.Catal_12_762 |
| PubMedSearch : Zhou_2022_ACS.Catal_12_762 |
| PubMedID: |
| Gene_locus related to this paper: 9actn-f1cla7 |
| Title : A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis - Aparicio_2000_Chem.Biol_7_895 |
| Author(s) : Aparicio JF , Fouces R , Mendes MV , Olivera N , Martin JF |
| Ref : Chemical Biology , 7 :895 , 2000 |
| Abstract : Aparicio_2000_Chem.Biol_7_895 |
| ESTHER : Aparicio_2000_Chem.Biol_7_895 |
| PubMedSearch : Aparicio_2000_Chem.Biol_7_895 |
| PubMedID: 11094342 |
| Gene_locus related to this paper: strna-PIMI , strna-PIMS4 |