Phenylacetate
General
Type : Acetate || Aryl ester
Chemical_Nomenclature : phenyl acetate
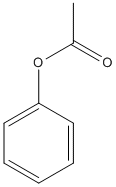
Canonical SMILES : CC(=O)OC1=CC=CC=C1
InChI : InChI=1S\/C8H8O2\/c1-7(9)10-8-5-3-2-4-6-8\/h2-6H,1H3
InChIKey : IPBVNPXQWQGGJP-UHFFFAOYSA-N
Other name(s) : PA, Phenol acetate, Acetylphenol, Acetic acid phenyl ester, Acetic acid, phenyl ester
Target
Families : Carb_B_Chordata, Hormone-sensitive_lipase_like, Canar_LipB
References (7)
| Title : Structure, biochemical characterization and analysis of the pleomorphism of carboxylesterase Cest-2923 from Lactobacillus plantarum WCFS1 - Benavente_2013_FEBS.J_280_6658 |
| Author(s) : Benavente R , Esteban-Torres M , Acebron I , de Las Rivas B , Munoz R , Alvarez Y , Mancheno JM |
| Ref : Febs J , 280 :6658 , 2013 |
| Abstract : Benavente_2013_FEBS.J_280_6658 |
| ESTHER : Benavente_2013_FEBS.J_280_6658 |
| PubMedSearch : Benavente_2013_FEBS.J_280_6658 |
| PubMedID: 24127688 |
| Gene_locus related to this paper: lacpl-LP.2923 |
| Title : Understanding the plasticity of the alpha\/beta hydrolase fold: lid swapping on the Candida antarctica lipase B results in chimeras with interesting biocatalytic properties - Skjot_2009_Chembiochem_10_520 |
| Author(s) : Skjot M , De Maria L , Chatterjee R , Svendsen A , Patkar SA , Ostergaard PR , Brask J |
| Ref : Chembiochem , 10 :520 , 2009 |
| Abstract : Skjot_2009_Chembiochem_10_520 |
| ESTHER : Skjot_2009_Chembiochem_10_520 |
| PubMedSearch : Skjot_2009_Chembiochem_10_520 |
| PubMedID: 19156649 |
| Gene_locus related to this paper: canar-LipB |
| Title : Electrostatic influence on the kinetics of ligand binding to acetylcholinesterase. Distinctions between active center ligands and fasciculin - Radic_1997_J.Biol.Chem_272_23265 |
| Author(s) : Radic Z , Kirchhoff PD , Quinn DM , McCammon JA , Taylor P |
| Ref : Journal of Biological Chemistry , 272 :23265 , 1997 |
| Abstract : Radic_1997_J.Biol.Chem_272_23265 |
| ESTHER : Radic_1997_J.Biol.Chem_272_23265 |
| PubMedSearch : Radic_1997_J.Biol.Chem_272_23265 |
| PubMedID: 9287336 |
| Title : Differentiation of esterases reacting with organophosphorus compounds - Reiner_1993_Chem.Biol.Interact_87_77 |
| Author(s) : Reiner E , Pavkovic E , Radic Z , Simeon-Rudolf V |
| Ref : Chemico-Biological Interactions , 87 :77 , 1993 |
| Abstract : Reiner_1993_Chem.Biol.Interact_87_77 |
| ESTHER : Reiner_1993_Chem.Biol.Interact_87_77 |
| PubMedSearch : Reiner_1993_Chem.Biol.Interact_87_77 |
| PubMedID: 8393750 |
| Title : Peripheral esterases in the rat: effects of classical inducers - McCracken_1993_Chem.Biol.Interact_87_183 |
| Author(s) : McCracken NW , Blain PG , Williams FM |
| Ref : Chemico-Biological Interactions , 87 :183 , 1993 |
| Abstract : McCracken_1993_Chem.Biol.Interact_87_183 |
| ESTHER : McCracken_1993_Chem.Biol.Interact_87_183 |
| PubMedSearch : McCracken_1993_Chem.Biol.Interact_87_183 |
| PubMedID: 8343974 |
| Title : Carboxylesterases in the respiratory tracts of rabbits, rats and Syrian hamsters - Dahl_1987_Toxicol.Lett_36_129 |
| Author(s) : Dahl AR , Miller SC , Petridou-Fischer J |
| Ref : Toxicol Lett , 36 :129 , 1987 |
| Abstract : Dahl_1987_Toxicol.Lett_36_129 |
| ESTHER : Dahl_1987_Toxicol.Lett_36_129 |
| PubMedSearch : Dahl_1987_Toxicol.Lett_36_129 |
| PubMedID: 3576643 |