Permethrin
General
Type : pyrethroid || Insecticide || Drug || Not A\/B H target || Phenoxyphenyl || Cyclopropane
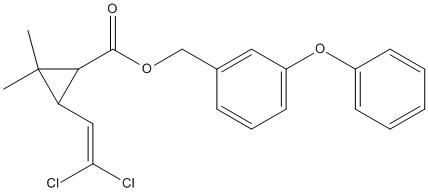
Chemical_Nomenclature : (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate
Canonical SMILES : CC1(C(C1C(=O)OCC2=CC(=CC=C2)OC3=CC=CC=C3)C=C(Cl)Cl)C
InChI : InChI=1S\/C21H20Cl2O3\/c1-21(2)17(12-18(22)23)19(21)20(24)25-13-14-7-6-10-16(11-14)26-15-8-4-3-5-9-15\/h3-12,17,19H,13H2,1-2H3
InChIKey : RLLPVAHGXHCWKJ-UHFFFAOYSA-N
Other name(s) : (1RS)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate, 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester, Permethrine, Ambushfog, Chinetrin, Efmethrin, Imperator, Indothrin, Outflank, Permasect, Perthrine, Ambush, Ectiban, Pounce, FMC 33297, BW-21-Z, Eksmin, Kafil, Pramex, Talcord, Nix, Dragnet
Target
Families : Carboxylesterase, Carb_B_Chordata, Carb_B_Arthropoda, Carb_B_Bacteria
References (16)
| Title : Molecular and functional characterization of three novel carboxylesterases in the detoxification of permethrin in the mosquito, Culex quinquefasciatus - Gong_2021_Insect.Sci__ |
| Author(s) : Gong Y , Li M , Li T , Liu N |
| Ref : Insect Sci , : , 2021 |
| Abstract : Gong_2021_Insect.Sci__ |
| ESTHER : Gong_2021_Insect.Sci__ |
| PubMedSearch : Gong_2021_Insect.Sci__ |
| PubMedID: 34048147 |
| Gene_locus related to this paper: culqu-b0xfi1 , culqu-b0xfi2 , culqu-b0xfi3 |
| Title : Development and Application of a Life-Stage Physiologically Based Pharmacokinetic (PBPK) Model to the Assessment of Internal Dose of Pyrethroids in Humans - Mallick_2020_Toxicol.Sci_173_86 |
| Author(s) : Mallick P , Moreau M , Song G , Efremenko AY , Pendse SN , Creek MR , Osimitz TG , Hines RN , Hinderliter P , Clewell HJ , Lake BG , Yoon M |
| Ref : Toxicol Sci , 173 :86 , 2020 |
| Abstract : Mallick_2020_Toxicol.Sci_173_86 |
| ESTHER : Mallick_2020_Toxicol.Sci_173_86 |
| PubMedSearch : Mallick_2020_Toxicol.Sci_173_86 |
| PubMedID: 31593217 |
| Title : Metabolism of deltamethrin and cis- and trans-permethrin by rat and human liver microsomes, liver cytosol and plasma preparations - Hedges_2019_Xenobiotica_49_388 |
| Author(s) : Hedges L , Brown S , Vardy A , Doyle E , Yoon M , Osimitz TG , Lake BG |
| Ref : Xenobiotica , 49 :388 , 2019 |
| Abstract : Hedges_2019_Xenobiotica_49_388 |
| ESTHER : Hedges_2019_Xenobiotica_49_388 |
| PubMedSearch : Hedges_2019_Xenobiotica_49_388 |
| PubMedID: 29537356 |
| Title : Metabolism of deltamethrin and cis- and trans-permethrin by human expressed cytochrome P450 and carboxylesterase enzymes - Hedges_2019_Xenobiotica_49_521 |
| Author(s) : Hedges L , Brown S , MacLeod AK , Vardy A , Doyle E , Song G , Moreau M , Yoon M , Osimitz TG , Lake BG |
| Ref : Xenobiotica , 49 :521 , 2019 |
| Abstract : Hedges_2019_Xenobiotica_49_521 |
| ESTHER : Hedges_2019_Xenobiotica_49_521 |
| PubMedSearch : Hedges_2019_Xenobiotica_49_521 |
| PubMedID: 29779438 |
| Title : Permethrin drastically affects the developmental cycle of the non-target slime mould Dictyostelium discoideum - Amaroli_2017_Chemosphere_193_1 |
| Author(s) : Amaroli A , Gallus L , Ferrando S |
| Ref : Chemosphere , 193 :1 , 2017 |
| Abstract : Amaroli_2017_Chemosphere_193_1 |
| ESTHER : Amaroli_2017_Chemosphere_193_1 |
| PubMedSearch : Amaroli_2017_Chemosphere_193_1 |
| PubMedID: 29121537 |
| Title : Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin - Yang_2009_Biochem.Pharmacol_77_238 |
| Author(s) : Yang D , Pearce RE , Wang X , Gaedigk R , Wan YJ , Yan B |
| Ref : Biochemical Pharmacology , 77 :238 , 2009 |
| Abstract : Yang_2009_Biochem.Pharmacol_77_238 |
| ESTHER : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedSearch : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedID: 18983829 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae) - Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| Author(s) : Zhang L , Gao X , Liang P |
| Ref : Pesticide Biochemistry and Physiology , 89 :65 , 2007 |
| Abstract : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| ESTHER : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedSearch : Zhang_2007_Pestic.Biochem.Physiol_89_65 |
| PubMedID: |
| Title : Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver and serum carboxylesterases - Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| Author(s) : Crow JA , Borazjani A , Potter PM , Ross MK |
| Ref : Toxicol Appl Pharmacol , 221 :1 , 2007 |
| Abstract : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| ESTHER : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| PubMedSearch : Crow_2007_Toxicol.Appl.Pharmacol_221_1 |
| PubMedID: 17442360 |
| Title : The in vitro metabolism of a pyrethroid insecticide, permethrin, and its hydrolysis products in rats - Nakamura_2007_Toxicology_235_176 |
| Author(s) : Nakamura Y , Sugihara K , Sone T , Isobe M , Ohta S , Kitamura S |
| Ref : Toxicology , 235 :176 , 2007 |
| Abstract : Nakamura_2007_Toxicology_235_176 |
| ESTHER : Nakamura_2007_Toxicology_235_176 |
| PubMedSearch : Nakamura_2007_Toxicology_235_176 |
| PubMedID: 17451859 |
| Title : Characterization of pyrethroid hydrolysis by the human liver carboxylesterases hCE-1 and hCE-2 - Nishi_2006_Arch.Biochem.Biophys_445_115 |
| Author(s) : Nishi K , Huang H , Kamita SG , Kim IH , Morisseau C , Hammock BD |
| Ref : Archives of Biochemistry & Biophysics , 445 :115 , 2006 |
| Abstract : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| ESTHER : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedSearch : Nishi_2006_Arch.Biochem.Biophys_445_115 |
| PubMedID: 16359636 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Hydrolysis of pyrethroids by carboxylesterases from Lucilia cuprina and Drosophila melanogaster with active sites modified by in vitro mutagenesis - Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| Author(s) : Heidari R , Devonshire AL , Campbell BE , Dorrian SJ , Oakeshott JG , Russell RJ |
| Ref : Insect Biochemistry & Molecular Biology , 35 :597 , 2005 |
| Abstract : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| ESTHER : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| PubMedSearch : Heidari_2005_Insect.Biochem.Mol.Biol_35_597 |
| PubMedID: 15857765 |
| Gene_locus related to this paper: drome-EST23aes07 , luccu-E3aest7 |
| Title : Identification, expression, and purification of a pyrethroid-hydrolyzing carboxylesterase from mouse liver microsomes - Stok_2004_J.Biol.Chem_279_29863 |
| Author(s) : Stok JE , Huang H , Jones PD , Wheelock CE , Morisseau C , Hammock BD |
| Ref : Journal of Biological Chemistry , 279 :29863 , 2004 |
| Abstract : Stok_2004_J.Biol.Chem_279_29863 |
| ESTHER : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedSearch : Stok_2004_J.Biol.Chem_279_29863 |
| PubMedID: 15123619 |
| Gene_locus related to this paper: mouse-Ces2a , mouse-Ces2e |
| Title : Isolation and identification of an esterase from a Mexican strain of Boophilus microplus (Acari: Ixodidae) - Pruett_2002_J.Econ.Entomol_95_1001 |
| Author(s) : Pruett JH , Guerrero FD , Hernandez R |
| Ref : J Econ Entomol , 95 :1001 , 2002 |
| Abstract : Pruett_2002_J.Econ.Entomol_95_1001 |
| ESTHER : Pruett_2002_J.Econ.Entomol_95_1001 |
| PubMedSearch : Pruett_2002_J.Econ.Entomol_95_1001 |
| PubMedID: 12403427 |
| Title : Influence of permethrin, diazinon and ivermectin treatments on insecticide resistance in the horn fly (Diptera: Muscidae) - Byford_1999_Int.J.Parasitol_29_125 |
| Author(s) : Byford RL , Craig ME , DeRouen SM , Kimball MD , Morrison DG , Wyatt WE , Foil LD |
| Ref : International Journal for Parasitology , 29 :125 , 1999 |
| Abstract : Byford_1999_Int.J.Parasitol_29_125 |
| ESTHER : Byford_1999_Int.J.Parasitol_29_125 |
| PubMedSearch : Byford_1999_Int.J.Parasitol_29_125 |
| PubMedID: 10048825 |
| Title : Permethrin Carboxylesterase Functions as Nonspecific Sequestration Proteins in the Hemolymph of Colorado Potato Beetle, - Lee_1998_Pestic.Biochem.Physiol_62_51 |
| Author(s) : Lee SH , Clark JM |
| Ref : Pesticide Biochemistry and Physiology , 62 :51 , 1998 |
| Abstract : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| ESTHER : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| PubMedSearch : Lee_1998_Pestic.Biochem.Physiol_62_51 |
| PubMedID: |
| Title : Insecticide susceptibility in mosquitoes (Diptera: Culicidae) from French Polynesia - Failloux_1994_J.Med.Entomol_31_639 |
| Author(s) : Failloux AB , Ung A , Raymond M , Pasteur N |
| Ref : Journal of Medical Entomology , 31 :639 , 1994 |
| Abstract : Failloux_1994_J.Med.Entomol_31_639 |
| ESTHER : Failloux_1994_J.Med.Entomol_31_639 |
| PubMedSearch : Failloux_1994_J.Med.Entomol_31_639 |
| PubMedID: 7966164 |