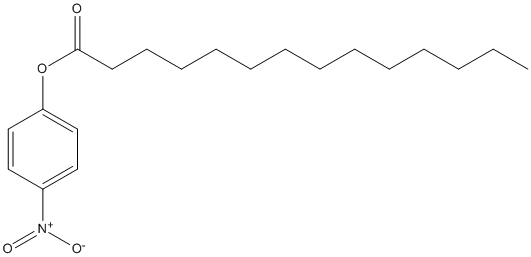
Paranitrophenyl-myristate
General
Type : pNP || Chromogen || Water-insoluble medium and long chain ester || Tetradecanoate
Chemical_Nomenclature : (4-nitrophenyl) tetradecanoate
Canonical SMILES : CCCCCCCCCCCCCC(=O)OC1=CC=C(C=C1)[N+](=O)[O-]
InChI : InChI=1S\/C20H31NO4\/c1-2-3-4-5-6-7-8-9-10-11-12-13-20(22)25-19-16-14-18(15-17-19)21(23)24\/h14-17H,2-13H2,1H3
InChIKey : ZBBNFJIVAHGZKA-UHFFFAOYSA-N
Other name(s) : pNP-C14, pNPM, p-NPM, 4-Nitrophenyl tetradecanoate, 4-Nitrophenyl myristate, p-NP tetradecanoate, p-NP myristate, pNP-tetradecanoate, pNP-myristate, P-Nitrophenyl myristate, Tetradecanoic acid 4-nitrophenyl ester, NSC122044
References (3)
| Title : The short form of the recombinant CAL-A-type lipase UM03410 from the smut fungus Ustilago maydis exhibits an inherent trans-fatty acid selectivity - Brundiek_2012_Appl.Microbiol.Biotechnol_94_141 |
| Author(s) : Brundiek H , Sass S , Evitt A , Kourist R , Bornscheuer UT |
| Ref : Applied Microbiology & Biotechnology , 94 :141 , 2012 |
| Abstract : Brundiek_2012_Appl.Microbiol.Biotechnol_94_141 |
| ESTHER : Brundiek_2012_Appl.Microbiol.Biotechnol_94_141 |
| PubMedSearch : Brundiek_2012_Appl.Microbiol.Biotechnol_94_141 |
| PubMedID: 22294433 |
| Gene_locus related to this paper: ustma-q4p903 |
| Title : Cloning, expression, and characterization of a cold-adapted lipase gene from an antarctic deep-sea psychrotrophic bacterium, Psychrobacter sp 7195 - Zhang_2007_J.Microbiol.Biotechnol_17_604 |
| Author(s) : Zhang J , Lin S , Zeng R |
| Ref : J Microbiol Biotechnol , 17 :604 , 2007 |
| Abstract : Zhang_2007_J.Microbiol.Biotechnol_17_604 |
| ESTHER : Zhang_2007_J.Microbiol.Biotechnol_17_604 |
| PubMedSearch : Zhang_2007_J.Microbiol.Biotechnol_17_604 |
| PubMedID: 18051271 |
| Gene_locus related to this paper: 9gamm-q2ktb4 |
| Title : A novel extracellular esterase from Bacillus subtilis and its conversion to a monoacylglycerol hydrolase - Eggert_2000_Eur.J.Biochem_267_6459 |
| Author(s) : Eggert T , Pencreac'h G , Douchet I , Verger R , Jaeger KE |
| Ref : European Journal of Biochemistry , 267 :6459 , 2000 |
| Abstract : Eggert_2000_Eur.J.Biochem_267_6459 |
| ESTHER : Eggert_2000_Eur.J.Biochem_267_6459 |
| PubMedSearch : Eggert_2000_Eur.J.Biochem_267_6459 |
| PubMedID: 11029590 |
| Gene_locus related to this paper: bacsu-LIPB |