Palmitoylcarnitine
General
Type : Fatty acid ester || Water-insoluble medium and long chain ester || Hexadecanoate || Palmitate
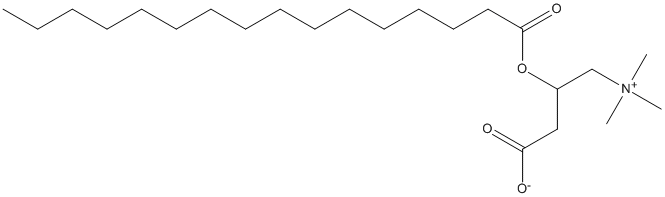
Chemical_Nomenclature : 3-hexadecanoyloxy-4-(trimethylazaniumyl)butanoate
Canonical SMILES : CCCCCCCCCCCCCCCC(=O)OC(CC(=O)[O-])C[N+](C)(C)C
InChI : InChI=1S\/C23H45NO4\/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-23(27)28-21(19-22(25)26)20-24(2,3)4\/h21H,5-20H2,1-4H3
InChIKey : XOMRRQXKHMYMOC-UHFFFAOYSA-N
Other name(s) : O-palmitoylcarnitine, O-hexadecanoylcarnitine, CHEBI:73067
Target
Families : Carb_B_Chordata
References (3)
| Title : Purification, molecular cloning, and functional expression of inducible liver acylcarnitine hydrolase in C57BL\/6 mouse, belonging to the carboxylesterase multigene family - Furihata_2003_Arch.Biochem.Biophys_416_101 |
| Author(s) : Furihata T , Hosokawa M , Nakata F , Satoh T , Chiba K |
| Ref : Archives of Biochemistry & Biophysics , 416 :101 , 2003 |
| Abstract : Furihata_2003_Arch.Biochem.Biophys_416_101 |
| ESTHER : Furihata_2003_Arch.Biochem.Biophys_416_101 |
| PubMedSearch : Furihata_2003_Arch.Biochem.Biophys_416_101 |
| PubMedID: 12859986 |
| Gene_locus related to this paper: mouse-Ces2c |
| Title : Subcellular localization of non-specific carboxylesterases, acylcarnitine hydrolase, monoacylglycerol lipase and palmitoyl-CoA hydrolase in rat liver - Mentlein_1988_Biochim.Biophys.Acta_964_319 |
| Author(s) : Mentlein R , Rix-Matzen H , Heymann E |
| Ref : Biochimica & Biophysica Acta , 964 :319 , 1988 |
| Abstract : Mentlein_1988_Biochim.Biophys.Acta_964_319 |
| ESTHER : Mentlein_1988_Biochim.Biophys.Acta_964_319 |
| PubMedSearch : Mentlein_1988_Biochim.Biophys.Acta_964_319 |
| PubMedID: 2894861 |
| Title : Specificity of two different purified acylcarnitine hydrolases from rat liver, their identity with other carboxylesterases, and their possible function - Mentlein_1985_Arch.Biochem.Biophys_240_801 |
| Author(s) : Mentlein R , Reuter G , Heymann E |
| Ref : Archives of Biochemistry & Biophysics , 240 :801 , 1985 |
| Abstract : Mentlein_1985_Arch.Biochem.Biophys_240_801 |
| ESTHER : Mentlein_1985_Arch.Biochem.Biophys_240_801 |
| PubMedSearch : Mentlein_1985_Arch.Biochem.Biophys_240_801 |
| PubMedID: 4026306 |