PAF-2-Thio
General
Type : Trimethylammonium || Choline ester
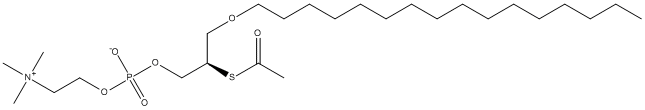
Chemical_Nomenclature : [(2R)-2-acetylsulfanyl-3-hexadecoxypropyl] 2-(trimethylazaniumyl)ethyl phosphate
Canonical SMILES : CCCCCCCCCCCCCCCCOCC(COP(=O)([O-])OCC[N+](C)(C)C)SC(=O)C
InChI : InChI=1S\/C26H54NO6PS\/c1-6-7-8-9-10-11-12-13-14-15-16-17-18-19-21-31-23-26(35-25(2)28)24-33-34(29,30)32-22-20-27(3,4)5\/h26H,6-24H2,1-5H3\/t26-\/m1\/s1
InChIKey : YPPZOKNKENLFTE-AREMUKBSSA-N
Other name(s) : 2-thio PAF, 1-O-Hexadecyl-2-deoxy-2-thio-S-acetyl-sn-glyceryl-3-phosphorylcholine, CHEBI:183981, HMS3649A13, HY-101263
MW : 539.74
Formula : C26H54NO6PS
CAS_number : 74389-68-7 || 96801-55-7
PubChem :
UniChem :
Iuphar :
Target
References (3)
| Title : Lipoprotein-Associated Phospholipase A2 Activity as Potential Biomarker of Vascular Dementia - Zuliani_2023_Antioxidants.(Basel)_12_597 |
| Author(s) : ZulianiZuliani G , Marsillach J , Trentini A , Rosta V , Cervellati C |
| Ref : Antioxidants (Basel) , 12 :597 , 2023 |
| Abstract : Zuliani_2023_Antioxidants.(Basel)_12_597 |
| ESTHER : Zuliani_2023_Antioxidants.(Basel)_12_597 |
| PubMedSearch : Zuliani_2023_Antioxidants.(Basel)_12_597 |
| PubMedID: 36978845 |
| Gene_locus related to this paper: human-PLA2G7 |
| Title : Catabolism of platelet-activating factor and its acyl analog. Differentiation of the activities of lysophospholipase and platelet- activating-factor acetylhydrolase - Aarsman_1991_Eur.J.Biochem_200_187 |
| Author(s) : Aarsman AJ , Neys FW , Van den Bosch H |
| Ref : European Journal of Biochemistry , 200 :187 , 1991 |
| Abstract : Aarsman_1991_Eur.J.Biochem_200_187 |
| ESTHER : Aarsman_1991_Eur.J.Biochem_200_187 |
| PubMedSearch : Aarsman_1991_Eur.J.Biochem_200_187 |
| PubMedID: 1879423 |
| Title : Structure-activity relationships for platelet-activating factor (PAF) and analogues reveal differences between PAF receptors on platelets and macrophages - Stewart_1991_J.Lipid.Mediat_4_299 |
| Author(s) : Stewart AG , Grigoriadis G |
| Ref : J Lipid Mediat , 4 :299 , 1991 |
| Abstract : Stewart_1991_J.Lipid.Mediat_4_299 |
| ESTHER : Stewart_1991_J.Lipid.Mediat_4_299 |
| PubMedSearch : Stewart_1991_J.Lipid.Mediat_4_299 |
| PubMedID: 1662547 |