Oseltamivir
General
Type : Pro-Drug || Drug || Not A\/B H target
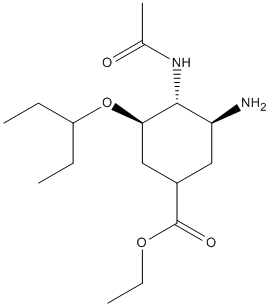
Chemical_Nomenclature : ethyl (3R,4R,5S)-4-acetamido-5-amino-3-pentan-3-yloxycyclohexene-1-carboxylate
Canonical SMILES : CCC(CC)OC1C=C(CC(C1NC(=O)C)N)C(=O)OCC
InChI : InChI=1S\/C16H28N2O4\/c1-5-12(6-2)22-14-9-11(16(20)21-7-3)8-13(17)15(14)18-10(4)19\/h9,12-15H,5-8,17H2,1-4H3,(H,18,19)\/t13-,14+,15+\/m0\/s1
InChIKey : VSZGPKBBMSAYNT-RRFJBIMHSA-N
Other name(s) : Tamvir, Tamiflu, Tamiflu-Free, GS-4104, (-)-oseltamivir
Target
Families : Carb_B_Chordata, BCHE
References (25)
| Title : Preliminary results on the uptake and biochemical response to water-exposure of Tamiflu(R) (oseltamivir phosphate) in two marine bivalves - Dallares_2019_J.Toxicol.Environ.Health.A__1 |
| Author(s) : Dallares S , Montemurro N , Perez S , Rodriguez-Sanchez N , Sole M |
| Ref : J Toxicol Environ Health A , :1 , 2019 |
| Abstract : Dallares_2019_J.Toxicol.Environ.Health.A__1 |
| ESTHER : Dallares_2019_J.Toxicol.Environ.Health.A__1 |
| PubMedSearch : Dallares_2019_J.Toxicol.Environ.Health.A__1 |
| PubMedID: 30669952 |
| Title : Evaluation of potential herb-drug interactions between oseltamivir and commonly used anti-influenza Chinese medicinal herbs - Zhang_2019_J.Ethnopharmacol__112097 |
| Author(s) : Zhang Y , Lyu C , Fong S , Wang Q , Li C , Ho NJ , Chan KS , Yan X , Zuo Z |
| Ref : J Ethnopharmacol , :112097 , 2019 |
| Abstract : Zhang_2019_J.Ethnopharmacol__112097 |
| ESTHER : Zhang_2019_J.Ethnopharmacol__112097 |
| PubMedSearch : Zhang_2019_J.Ethnopharmacol__112097 |
| PubMedID: 31325600 |
| Title : Age-Dependent Absolute Abundance of Hepatic Carboxylesterases (CES1 and CES2) by LC-MS\/MS Proteomics: Application to PBPK Modeling of Oseltamivir In Vivo Pharmacokinetics in Infants - Boberg_2017_Drug.Metab.Dispos_45_216 |
| Author(s) : Boberg M , Vrana M , Mehrotra A , Pearce RE , Gaedigk A , Bhatt DK , Leeder JS , Prasad B |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 45 :216 , 2017 |
| Abstract : Boberg_2017_Drug.Metab.Dispos_45_216 |
| ESTHER : Boberg_2017_Drug.Metab.Dispos_45_216 |
| PubMedSearch : Boberg_2017_Drug.Metab.Dispos_45_216 |
| PubMedID: 27895113 |
| Gene_locus related to this paper: human-CES2 |
| Title : Pharmacokinetic Modeling and Monte Carlo Simulation to Predict Interindividual Variability in Human Exposure to Oseltamivir and Its Active Metabolite, Ro 64-0802 - Ito_2017_AAPS.J_19_286 |
| Author(s) : Ito M , Kusuhara H , Ose A , Kondo T , Tanabe K , Nakayama H , Horita S , Fujita T , Sugiyama Y |
| Ref : AAPS J , 19 :286 , 2017 |
| Abstract : Ito_2017_AAPS.J_19_286 |
| ESTHER : Ito_2017_AAPS.J_19_286 |
| PubMedSearch : Ito_2017_AAPS.J_19_286 |
| PubMedID: 27800573 |
| Title : Effects of dexamethasone coadministered with oseltamivir on the pharmacokinetics of oseltamivir in healthy volunteers - Jang_2017_Drug.Des.Devel.Ther_11_705 |
| Author(s) : Jang K , Kim MK , Oh J , Lee S , Cho JY , Yu KS , Choi TK , Lee SH , Lim KS |
| Ref : Drug Des Devel Ther , 11 :705 , 2017 |
| Abstract : Jang_2017_Drug.Des.Devel.Ther_11_705 |
| ESTHER : Jang_2017_Drug.Des.Devel.Ther_11_705 |
| PubMedSearch : Jang_2017_Drug.Des.Devel.Ther_11_705 |
| PubMedID: 28331290 |
| Title : The novel carboxylesterase 1 variant c.662A>G may decrease the bioactivation of oseltamivir in humans - Oh_2017_PLoS.One_12_e0176320 |
| Author(s) : Oh J , Lee S , Lee H , Cho JY , Yoon SH , Jang IJ , Yu KS , Lim KS |
| Ref : PLoS ONE , 12 :e0176320 , 2017 |
| Abstract : Oh_2017_PLoS.One_12_e0176320 |
| ESTHER : Oh_2017_PLoS.One_12_e0176320 |
| PubMedSearch : Oh_2017_PLoS.One_12_e0176320 |
| PubMedID: 28437488 |
| Title : Association of Oseltamivir Activation with Gender and Carboxylesterase 1 Genetic Polymorphisms - Shi_2016_Basic.Clin.Pharmacol.Toxicol_119_555 |
| Author(s) : Shi J , Wang X , Eyler RF , Liang Y , Liu L , Mueller BA , Zhu HJ |
| Ref : Basic Clin Pharmacol Toxicol , 119 :555 , 2016 |
| Abstract : Shi_2016_Basic.Clin.Pharmacol.Toxicol_119_555 |
| ESTHER : Shi_2016_Basic.Clin.Pharmacol.Toxicol_119_555 |
| PubMedSearch : Shi_2016_Basic.Clin.Pharmacol.Toxicol_119_555 |
| PubMedID: 27228223 |
| Gene_locus related to this paper: human-CES1 |
| Title : Substrate-Competitive Activity-Based Profiling of Ester Prodrug Activating Enzymes - Xu_2015_Mol.Pharm_12_3399 |
| Author(s) : Xu H , Majmudar JD , Davda D , Ghanakota P , Kim KH , Carlson HA , Showalter HD , Martin BR , Amidon GL |
| Ref : Mol Pharm , 12 :3399 , 2015 |
| Abstract : Xu_2015_Mol.Pharm_12_3399 |
| ESTHER : Xu_2015_Mol.Pharm_12_3399 |
| PubMedSearch : Xu_2015_Mol.Pharm_12_3399 |
| PubMedID: 26262434 |
| Gene_locus related to this paper: human-CES1 |
| Title : Comparison of substrate specificity among human arylacetamide deacetylase and carboxylesterases - Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| Author(s) : Fukami T , Kariya M , Kurokawa T , Iida A , Nakajima M |
| Ref : Eur J Pharm Sci , 78 :47 , 2015 |
| Abstract : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| ESTHER : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| PubMedSearch : Fukami_2015_Eur.J.Pharm.Sci_78_47 |
| PubMedID: 26164127 |
| Gene_locus related to this paper: human-AADAC , human-CES1 , human-CES2 |
| Title : Effects of alcohol on human carboxylesterase drug metabolism - Parker_2015_Clin.Pharmacokinet_54_627 |
| Author(s) : Parker RB , Hu ZY , Meibohm B , Laizure SC |
| Ref : Clinical Pharmacokinetics , 54 :627 , 2015 |
| Abstract : Parker_2015_Clin.Pharmacokinet_54_627 |
| ESTHER : Parker_2015_Clin.Pharmacokinet_54_627 |
| PubMedSearch : Parker_2015_Clin.Pharmacokinet_54_627 |
| PubMedID: 25511794 |
| Title : Physiologically based pharmacokinetic modeling of impaired carboxylesterase-1 activity: effects on oseltamivir disposition - Hu_2014_Clin.Pharmacokinet_53_825 |
| Author(s) : Hu ZY , Edginton AN , Laizure SC , Parker RB |
| Ref : Clinical Pharmacokinetics , 53 :825 , 2014 |
| Abstract : Hu_2014_Clin.Pharmacokinet_53_825 |
| ESTHER : Hu_2014_Clin.Pharmacokinet_53_825 |
| PubMedSearch : Hu_2014_Clin.Pharmacokinet_53_825 |
| PubMedID: 25103325 |
| Title : Carboxylesterase 1 (CES1) genetic polymorphisms and oseltamivir activation - |
| Author(s) : Zhu HJ , Markowitz JS |
| Ref : European Journal of Clinical Pharmacology , 69 :733 , 2013 |
| PubMedID: 22847618 |
| Title : The effect of carboxylesterase 1 (CES1) polymorphisms on the pharmacokinetics of oseltamivir in humans - Suzaki_2013_Eur.J.Clin.Pharmacol_69_21 |
| Author(s) : Suzaki Y , Uemura N , Takada M , Ohyama T , Itohda A , Morimoto T , Imai H , Hamasaki H , Inano A , Hosokawa M , Tateishi M , Ohashi K |
| Ref : European Journal of Clinical Pharmacology , 69 :21 , 2013 |
| Abstract : Suzaki_2013_Eur.J.Clin.Pharmacol_69_21 |
| ESTHER : Suzaki_2013_Eur.J.Clin.Pharmacol_69_21 |
| PubMedSearch : Suzaki_2013_Eur.J.Clin.Pharmacol_69_21 |
| PubMedID: 22673926 |
| Title : Carboxylesterase 1 polymorphism impairs oseltamivir bioactivation in humans - Tarkiainen_2012_Clin.Pharmacol.Ther_92_68 |
| Author(s) : Tarkiainen EK , Backman JT , Neuvonen M , Neuvonen PJ , Schwab M , Niemi M |
| Ref : Clinical Pharmacology & Therapeutics , 92 :68 , 2012 |
| Abstract : Tarkiainen_2012_Clin.Pharmacol.Ther_92_68 |
| ESTHER : Tarkiainen_2012_Clin.Pharmacol.Ther_92_68 |
| PubMedSearch : Tarkiainen_2012_Clin.Pharmacol.Ther_92_68 |
| PubMedID: 22588607 |
| Gene_locus related to this paper: human-CES1 |
| Title : Surge in expression of carboxylesterase 1 during the post-neonatal stage enables a rapid gain of the capacity to activate the anti-influenza prodrug oseltamivir - Shi_2011_J.Infect.Dis_203_937 |
| Author(s) : Shi D , Yang D , Prinssen EP , Davies BE , Yan B |
| Ref : J Infect Dis , 203 :937 , 2011 |
| Abstract : Shi_2011_J.Infect.Dis_203_937 |
| ESTHER : Shi_2011_J.Infect.Dis_203_937 |
| PubMedSearch : Shi_2011_J.Infect.Dis_203_937 |
| PubMedID: 21402544 |
| Title : The effect of natural health products and traditional medicines on the activity of human hepatic microsomal-mediated metabolism of oseltamivir - Liu_2010_J.Pharm.Pharm.Sci_13_43 |
| Author(s) : Liu R , Tam TW , Mao J , Saleem A , Krantis A , Arnason JT , Foster BC |
| Ref : J Pharm Pharm Sci , 13 :43 , 2010 |
| Abstract : Liu_2010_J.Pharm.Pharm.Sci_13_43 |
| ESTHER : Liu_2010_J.Pharm.Pharm.Sci_13_43 |
| PubMedSearch : Liu_2010_J.Pharm.Pharm.Sci_13_43 |
| PubMedID: 20456830 |
| Title : Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin - Yang_2009_Biochem.Pharmacol_77_238 |
| Author(s) : Yang D , Pearce RE , Wang X , Gaedigk R , Wan YJ , Yan B |
| Ref : Biochemical Pharmacology , 77 :238 , 2009 |
| Abstract : Yang_2009_Biochem.Pharmacol_77_238 |
| ESTHER : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedSearch : Yang_2009_Biochem.Pharmacol_77_238 |
| PubMedID: 18983829 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Studies on the influence of esterase inhibitor to the pharmacokinetic profiles of oseltamivir and oseltamivir carboxylate in rats using an improved LC\/MS\/MS method - Chang_2009_Biomed.Chromatogr_23_852 |
| Author(s) : Chang Q , Chow MS , Zuo Z |
| Ref : Biomedical Chromatography , 23 :852 , 2009 |
| Abstract : Chang_2009_Biomed.Chromatogr_23_852 |
| ESTHER : Chang_2009_Biomed.Chromatogr_23_852 |
| PubMedSearch : Chang_2009_Biomed.Chromatogr_23_852 |
| PubMedID: 19353695 |
| Title : Activation of the antiviral prodrug oseltamivir is impaired by two newly identified carboxylesterase 1 variants - Zhu_2009_Drug.Metab.Dispos_37_264 |
| Author(s) : Zhu HJ , Markowitz JS |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 37 :264 , 2009 |
| Abstract : Zhu_2009_Drug.Metab.Dispos_37_264 |
| ESTHER : Zhu_2009_Drug.Metab.Dispos_37_264 |
| PubMedSearch : Zhu_2009_Drug.Metab.Dispos_37_264 |
| PubMedID: 19022936 |
| Gene_locus related to this paper: human-CES1 |
| Title : Pharmacokinetics of high-dose oseltamivir in healthy volunteers - Wattanagoon_2009_Antimicrob.Agents.Chemother_53_945 |
| Author(s) : Wattanagoon Y , Stepniewska K , Lindegardh N , Pukrittayakamee S , Silachamroon U , Piyaphanee W , Singtoroj T , Hanpithakpong W , Davies G , Tarning J , Pongtavornpinyo W , Fukuda C , Singhasivanon P , Day NP , White NJ |
| Ref : Antimicrobial Agents & Chemotherapy , 53 :945 , 2009 |
| Abstract : Wattanagoon_2009_Antimicrob.Agents.Chemother_53_945 |
| ESTHER : Wattanagoon_2009_Antimicrob.Agents.Chemother_53_945 |
| PubMedSearch : Wattanagoon_2009_Antimicrob.Agents.Chemother_53_945 |
| PubMedID: 19104028 |
| Title : Oseltamivir, an anti-influenza virus drug, produces hypothermia in mice - Ono_2008_Biol.Pharm.Bull_31_638 |
| Author(s) : Ono H , Nagano Y , Matsunami N , Sugiyama S , Yamamoto S , Tanabe M |
| Ref : Biol Pharm Bull , 31 :638 , 2008 |
| Abstract : Ono_2008_Biol.Pharm.Bull_31_638 |
| ESTHER : Ono_2008_Biol.Pharm.Bull_31_638 |
| PubMedSearch : Ono_2008_Biol.Pharm.Bull_31_638 |
| PubMedID: 18379055 |
| Title : New, efficient synthesis of oseltamivir phosphate (Tamiflu) via enzymatic desymmetrization of a meso-1,3-cyclohexanedicarboxylic acid diester - Zutter_2008_J.Org.Chem_73_4895 |
| Author(s) : Zutter U , Iding H , Spurr P , Wirz B |
| Ref : J Org Chem , 73 :4895 , 2008 |
| Abstract : Zutter_2008_J.Org.Chem_73_4895 |
| ESTHER : Zutter_2008_J.Org.Chem_73_4895 |
| PubMedSearch : Zutter_2008_J.Org.Chem_73_4895 |
| PubMedID: 18517254 |
| Title : Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2 - Yang_2007_Mol.Pharmacol_72_686 |
| Author(s) : Yang J , Shi D , Yang D , Song X , Yan B |
| Ref : Molecular Pharmacology , 72 :686 , 2007 |
| Abstract : Yang_2007_Mol.Pharmacol_72_686 |
| ESTHER : Yang_2007_Mol.Pharmacol_72_686 |
| PubMedSearch : Yang_2007_Mol.Pharmacol_72_686 |
| PubMedID: 17537833 |
| Gene_locus related to this paper: human-CES1 , human-CES2 |
| Title : Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel - Shi_2006_J.Pharmacol.Exp.Ther_319_1477 |
| Author(s) : Shi D , Yang J , Yang D , LeCluyse EL , Black C , You L , Akhlaghi F , Yan B |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 319 :1477 , 2006 |
| Abstract : Shi_2006_J.Pharmacol.Exp.Ther_319_1477 |
| ESTHER : Shi_2006_J.Pharmacol.Exp.Ther_319_1477 |
| PubMedSearch : Shi_2006_J.Pharmacol.Exp.Ther_319_1477 |
| PubMedID: 16966469 |
| Title : Rapid degradation of oseltamivir phosphate in clinical samples by plasma esterases - Lindegardh_2006_Antimicrob.Agents.Chemother_50_3197 |
| Author(s) : Lindegardh N , Davies GR , Tran TH , Farrar J , Singhasivanon P , Day NP , White NJ |
| Ref : Antimicrobial Agents & Chemotherapy , 50 :3197 , 2006 |
| Abstract : Lindegardh_2006_Antimicrob.Agents.Chemother_50_3197 |
| ESTHER : Lindegardh_2006_Antimicrob.Agents.Chemother_50_3197 |
| PubMedSearch : Lindegardh_2006_Antimicrob.Agents.Chemother_50_3197 |
| PubMedID: 16940130 |