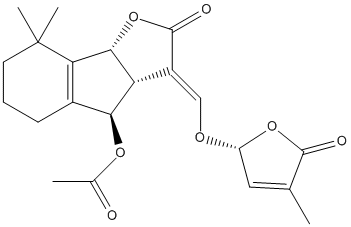
Orobanchyl-acetate
General
Type : Butenolide || Strigolactone receptors ligand
Chemical_Nomenclature : [(3E,3aR,4R,8bR)-8,8-dimethyl-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxymethylidene]-2-oxo-3a,4,5,6,7,8b-hexahydroindeno[1,2-b]furan-4-yl] acetate
Canonical SMILES : CC1=CC(OC1=O)OC=C2C3C(C4=C(C3OC(=O)C)CCCC4(C)C)OC2=O
InChI : InChI=1S\/C21H24O7\/c1-10-8-14(27-19(10)23)25-9-13-15-17(26-11(2)22)12-6-5-7-21(3,4)16(12)18(15)28-20(13)24\/h8-9,14-15,17-18H,5-7H2,1-4H3\/b13-9+\/t14-,15-,17+,18-\/m1\/s1
InChIKey : DLRIUVHQJRZTMZ-PLPBJLQDSA-N
Other name(s) : Orobanchyl acetate, KDR5EQ1GKY, UNII-KDR5EQ1GKY, C09059, SCHEMBL13857722
MW : 388.4
Formula : C21H24O7
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : RsbQ-like
References (12)
| Title : Characterization of a Chickpea Mutant Resistant to Phelipanche aegyptiaca Pers. and Orobanche crenata Forsk - Galili_2021_Plants.(Basel)_10_ |
| Author(s) : Galili S , Hershenhorn J , Smirnov E , Yoneyama K , Xie X , Amir-Segev O , Bellalou A , Dor E |
| Ref : Plants (Basel) , 10 : , 2021 |
| Abstract : Galili_2021_Plants.(Basel)_10_ |
| ESTHER : Galili_2021_Plants.(Basel)_10_ |
| PubMedSearch : Galili_2021_Plants.(Basel)_10_ |
| PubMedID: 34961023 |
| Title : A new UHPLC-MS\/MS method for the direct determination of strigolactones in root exudates and extracts - Rial_2019_Phytochem.Anal_30_110 |
| Author(s) : Rial C , Varela RM , Molinillo JMG , Lopez-Raez JA , Macias FA |
| Ref : Phytochem Anal , 30 :110 , 2019 |
| Abstract : Rial_2019_Phytochem.Anal_30_110 |
| ESTHER : Rial_2019_Phytochem.Anal_30_110 |
| PubMedSearch : Rial_2019_Phytochem.Anal_30_110 |
| PubMedID: 30280444 |
| Title : Validated Method for Strigolactone Quantification by Ultra High-Performance Liquid Chromatography - Electrospray Ionisation Tandem Mass Spectrometry Using Novel Deuterium Labelled Standards - Boutet-Mercey_2018_Phytochem.Anal_29_59 |
| Author(s) : Boutet-Mercey S , Perreau F , Roux A , Clave G , Pillot JP , Schmitz-Afonso I , Touboul D , Mouille G , Rameau C , Boyer FD |
| Ref : Phytochem Anal , 29 :59 , 2018 |
| Abstract : Boutet-Mercey_2018_Phytochem.Anal_29_59 |
| ESTHER : Boutet-Mercey_2018_Phytochem.Anal_29_59 |
| PubMedSearch : Boutet-Mercey_2018_Phytochem.Anal_29_59 |
| PubMedID: 28851101 |
| Title : Photomodulation of strigolactone biosynthesis and accumulation during sunflower seedling growth - Bharti_2015_Plant.Signal.Behav_10_e1049792 |
| Author(s) : Bharti N , Tripathi S , Bhatla SC |
| Ref : Plant Signal Behav , 10 :e1049792 , 2015 |
| Abstract : Bharti_2015_Plant.Signal.Behav_10_e1049792 |
| ESTHER : Bharti_2015_Plant.Signal.Behav_10_e1049792 |
| PubMedSearch : Bharti_2015_Plant.Signal.Behav_10_e1049792 |
| PubMedID: 26252191 |
| Title : Low strigolactone root exudation: a novel mechanism of broomrape (Orobanche and Phelipanche spp.) resistance available for faba bean breeding - Fernandez-Aparicio_2014_J.Agric.Food.Chem_62_7063 |
| Author(s) : Fernandez-Aparicio M , Kisugi T , Xie X , Rubiales D , Yoneyama K |
| Ref : Journal of Agricultural and Food Chemistry , 62 :7063 , 2014 |
| Abstract : Fernandez-Aparicio_2014_J.Agric.Food.Chem_62_7063 |
| ESTHER : Fernandez-Aparicio_2014_J.Agric.Food.Chem_62_7063 |
| PubMedSearch : Fernandez-Aparicio_2014_J.Agric.Food.Chem_62_7063 |
| PubMedID: 24974726 |
| Title : Confirming stereochemical structures of strigolactones produced by rice and tobacco - Xie_2013_Mol.Plant_6_153 |
| Author(s) : Xie X , Yoneyama K , Kisugi T , Uchida K , Ito S , Akiyama K , Hayashi H , Yokota T , Nomura T |
| Ref : Mol Plant , 6 :153 , 2013 |
| Abstract : Xie_2013_Mol.Plant_6_153 |
| ESTHER : Xie_2013_Mol.Plant_6_153 |
| PubMedSearch : Xie_2013_Mol.Plant_6_153 |
| PubMedID: 23204500 |
| Title : Tomato strigolactones: a more detailed look - Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| Author(s) : Kohlen W , Charnikhova T , Bours R , Lopez-Raez JA , Bouwmeester H |
| Ref : Plant Signal Behav , 8 :e22785 , 2013 |
| Abstract : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| ESTHER : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| PubMedSearch : Kohlen_2013_Plant.Signal.Behav_8_e22785 |
| PubMedID: 23221743 |
| Title : Ent-2'-epi-Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover - Ueno_2011_J.Agric.Food.Chem_59_10485 |
| Author(s) : Ueno K , Nomura S , Muranaka S , Mizutani M , Takikawa H , Sugimoto Y |
| Ref : Journal of Agricultural and Food Chemistry , 59 :10485 , 2011 |
| Abstract : Ueno_2011_J.Agric.Food.Chem_59_10485 |
| ESTHER : Ueno_2011_J.Agric.Food.Chem_59_10485 |
| PubMedSearch : Ueno_2011_J.Agric.Food.Chem_59_10485 |
| PubMedID: 21899364 |
| Title : Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis - Kohlen_2011_Plant.Physiol_155_974 |
| Author(s) : Kohlen W , Charnikhova T , Liu Q , Bours R , Domagalska MA , Beguerie S , Verstappen F , Leyser O , Bouwmeester H , Ruyter-Spira C |
| Ref : Plant Physiol , 155 :974 , 2011 |
| Abstract : Kohlen_2011_Plant.Physiol_155_974 |
| ESTHER : Kohlen_2011_Plant.Physiol_155_974 |
| PubMedSearch : Kohlen_2011_Plant.Physiol_155_974 |
| PubMedID: 21119045 |
| Title : Lupin pyranoisoflavones inhibiting hyphal development in arbuscular mycorrhizal fungi - Akiyama_2010_Phytochemistry_71_1865 |
| Author(s) : Akiyama K , Tanigawa F , Kashihara T , Hayashi H |
| Ref : Phytochemistry , 71 :1865 , 2010 |
| Abstract : Akiyama_2010_Phytochemistry_71_1865 |
| ESTHER : Akiyama_2010_Phytochemistry_71_1865 |
| PubMedSearch : Akiyama_2010_Phytochemistry_71_1865 |
| PubMedID: 20813384 |
| Title : 7-Oxoorobanchyl acetate and 7-Oxoorobanchol as germination stimulants for root parasitic plants from flax (Linum usitatissimum) - Xie_2009_Biosci.Biotechnol.Biochem_73_1367 |
| Author(s) : Xie X , Yoneyama K , Kurita JY , Harada Y , Yamada Y , Takeuchi Y |
| Ref : Biosci Biotechnol Biochem , 73 :1367 , 2009 |
| Abstract : Xie_2009_Biosci.Biotechnol.Biochem_73_1367 |
| ESTHER : Xie_2009_Biosci.Biotechnol.Biochem_73_1367 |
| PubMedSearch : Xie_2009_Biosci.Biotechnol.Biochem_73_1367 |
| PubMedID: 19502732 |
| Title : Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants - Yoneyama_2008_New.Phytol_179_484 |
| Author(s) : Yoneyama K , Xie X , Sekimoto H , Takeuchi Y , Ogasawara S , Akiyama K , Hayashi H |
| Ref : New Phytol , 179 :484 , 2008 |
| Abstract : Yoneyama_2008_New.Phytol_179_484 |
| ESTHER : Yoneyama_2008_New.Phytol_179_484 |
| PubMedSearch : Yoneyama_2008_New.Phytol_179_484 |
| PubMedID: 19086293 |