Oleoyl-CoA
General
Type : Natural || CoA || Aminopurin
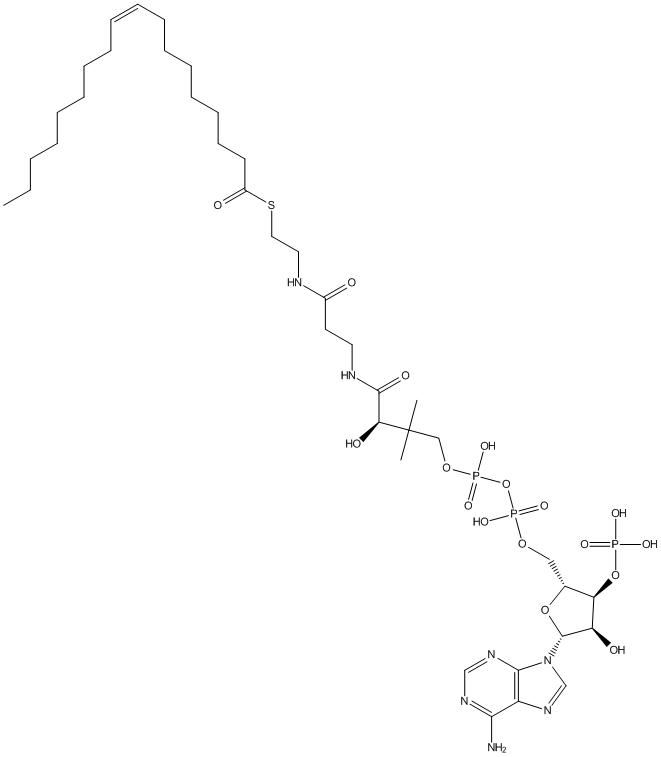
Chemical_Nomenclature : S-[2-[3-[[(2R)-4-[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-phosphonooxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]oxy-2-hydroxy-3,3-dimethylbutanoyl]amino]propanoylamino]ethyl] (Z)-octadec-9-enethioate
Canonical SMILES : CCCCCCCCC=CCCCCCCCC(=O)SCCNC(=O)CCNC(=O)C(C(C)(C)COP(=O)(O)OP(=O)(O)OCC1C(C(C(O1)N2C=NC3=C2N=CN=C3N)O)OP(=O)(O)O)O
InChI : InChI=1S\/C39H68N7O17P3S\/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-30(48)67-23-22-41-29(47)20-21-42-37(51)34(50)39(2,3)25-60-66(57,58)63-65(55,56)59-24-28-33(62-64(52,53)54)32(49)38(61-28)46-27-45-31-35(40)43-26-44-36(31)46\/h11-12,26-28,32-34,38,49-50H,4-10,13-25H2,1-3H3,(H,41,47)(H,42,51)(H,55,56)(H,57,58)(H2,40,43,44)(H2,52,53,54)\/b12-11-\/t28-,32-,33-,34+,38-\/m1\/s1
InChIKey : XDUHQPOXLUAVEE-BPMMELMSSA-N
Other name(s) : oleoyl-coenzyme A, S-Oleoylcoenzyme A, cis-9-Octadecenoyl-CoA, oleoyl-CoA, 1716-06-9, (9Z)-octadec-9-enoyl-CoA, Oleyl coenzyme A, Oleyl-CoA, S-oleoyl-CoA
Target
Families : Carboxylesterase, Carb_B_Chordata
References (2)
| Title : Mammalian carboxylesterases: from drug targets to protein therapeutics - Redinbo_2005_Drug.Discov.Today_10_313 |
| Author(s) : Redinbo MR , Potter PM |
| Ref : Drug Discov Today , 10 :313 , 2005 |
| Abstract : Redinbo_2005_Drug.Discov.Today_10_313 |
| ESTHER : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedSearch : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedID: 15749280 |
| Title : Purification, cloning, and expression of a human enzyme with acyl coenzyme A: cholesterol acyltransferase activity, which is identical to liver carboxylesterase - Becker_1994_Arterioscler.Thromb_14_1346 |
| Author(s) : Becker A , Bottcher A , Lackner KJ , Fehringer P , Notka F , Aslanidis C , Schmitz G |
| Ref : Arterioscler Thromb , 14 :1346 , 1994 |
| Abstract : Becker_1994_Arterioscler.Thromb_14_1346 |
| ESTHER : Becker_1994_Arterioscler.Thromb_14_1346 |
| PubMedSearch : Becker_1994_Arterioscler.Thromb_14_1346 |
| PubMedID: 8049197 |
| Gene_locus related to this paper: human-CES1 , pig-EST1 |