Octanoyl-ghrelin
General
Type : Acyl peptide
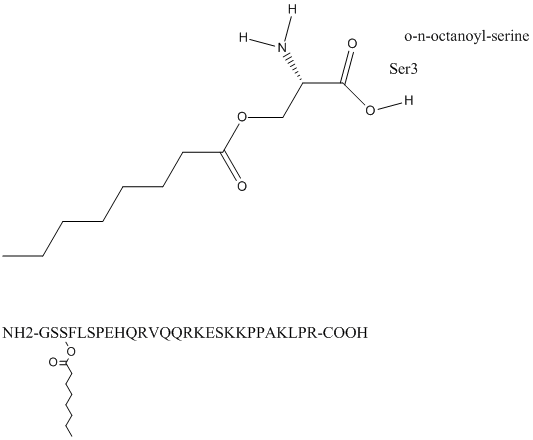
Chemical_Nomenclature : octanoyl ghrelin\; [O-n-octanoyl-Ser3]-human ghrelin
Canonical SMILES :
InChI :
InChIKey : BGHSOEHUOOAYMY-JTZMCQEISA-N
Other name(s) : Acyl-ghrelin, Gastric MLTRP, Motilin-related peptide, Human ghrelin, [Des-octanoyl]-Ghrelin (human)
MW :
Formula :
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : BCHE, Pectinacetylesterase-Notum
References (13)
| Title : Notum Deacylates Octanoylated Ghrelin - Zhao_2021_Mol.Metab__101201 |
| Author(s) : Zhao Y , Schuhmacher LN , Roberts M , Kakugawa S , Bineva-Todd G , Howell S , O'Reilly N , Perret C , Snijders AP , Vincent JP , Jones EY |
| Ref : Mol Metab , :101201 , 2021 |
| Abstract : Zhao_2021_Mol.Metab__101201 |
| ESTHER : Zhao_2021_Mol.Metab__101201 |
| PubMedSearch : Zhao_2021_Mol.Metab__101201 |
| PubMedID: 33647468 |
| Gene_locus related to this paper: human-NOTUM |
| Title : Ghrelin Octanoylation Is Completely Stabilized in Biological Samples by Alkyl Fluorophosphonates - McGovern-Gooch_2016_Endocrinology_157_4330 |
| Author(s) : McGovern-Gooch KR , Rodrigues T , Darling JE , Sieburg MA , Abizaid A , Hougland JL |
| Ref : Endocrinology , 157 :4330 , 2016 |
| Abstract : McGovern-Gooch_2016_Endocrinology_157_4330 |
| ESTHER : McGovern-Gooch_2016_Endocrinology_157_4330 |
| PubMedSearch : McGovern-Gooch_2016_Endocrinology_157_4330 |
| PubMedID: 27623288 |
| Title : Plasma butyrylcholinesterase regulates ghrelin to control aggression - Chen_2015_Proc.Natl.Acad.Sci.U.S.A_112_2251 |
| Author(s) : Chen VP , Gao Y , Geng L , Parks RJ , Pang YP , Brimijoin S |
| Ref : Proc Natl Acad Sci U S A , 112 :2251 , 2015 |
| Abstract : Chen_2015_Proc.Natl.Acad.Sci.U.S.A_112_2251 |
| ESTHER : Chen_2015_Proc.Natl.Acad.Sci.U.S.A_112_2251 |
| PubMedSearch : Chen_2015_Proc.Natl.Acad.Sci.U.S.A_112_2251 |
| PubMedID: 25646463 |
| Title : Pure human butyrylcholinesterase hydrolyzes octanoyl ghrelin to desacyl ghrelin - Schopfer_2015_Gen.Comp.Endocrinol_224_61 |
| Author(s) : Schopfer LM , Lockridge O , Brimijoin S |
| Ref : General & Comparative Endocrinology , 224 :61 , 2015 |
| Abstract : Schopfer_2015_Gen.Comp.Endocrinol_224_61 |
| ESTHER : Schopfer_2015_Gen.Comp.Endocrinol_224_61 |
| PubMedSearch : Schopfer_2015_Gen.Comp.Endocrinol_224_61 |
| PubMedID: 26073531 |
| Title : Radiometric assay of ghrelin hydrolase activity and (3)H-ghrelin distribution into mouse tissues - Chen_2015_Biochem.Pharmacol_98_732 |
| Author(s) : Chen VP , Gao Y , Geng L , Brimijoin S |
| Ref : Biochemical Pharmacology , 98 :732 , 2015 |
| Abstract : Chen_2015_Biochem.Pharmacol_98_732 |
| ESTHER : Chen_2015_Biochem.Pharmacol_98_732 |
| PubMedSearch : Chen_2015_Biochem.Pharmacol_98_732 |
| PubMedID: 26514871 |
| Title : Kinetic characterization of a cocaine hydrolase engineered from mouse butyrylcholinesterase - Chen_2015_Biochem.J_466_243 |
| Author(s) : Chen X , Huang X , Geng L , Xue L , Hou S , Zheng X , Brimijoin S , Zheng F , Zhan CG |
| Ref : Biochemical Journal , 466 :243 , 2015 |
| Abstract : Chen_2015_Biochem.J_466_243 |
| ESTHER : Chen_2015_Biochem.J_466_243 |
| PubMedSearch : Chen_2015_Biochem.J_466_243 |
| PubMedID: 25486543 |
| Gene_locus related to this paper: human-BCHE |
| Title : Obesity and variants of the GHRL (ghrelin) and BCHE (butyrylcholinesterase) genes - Dantas_2011_Genet.Mol.Biol_34_205 |
| Author(s) : Dantas VG , Furtado-Alle L , de Souza RLR , Chautard-Freire-Maia EA |
| Ref : Genet Mol Biol , 34 :205 , 2011 |
| Abstract : Dantas_2011_Genet.Mol.Biol_34_205 |
| ESTHER : Dantas_2011_Genet.Mol.Biol_34_205 |
| PubMedSearch : Dantas_2011_Genet.Mol.Biol_34_205 |
| PubMedID: 21734817 |
| Title : High-fat diet with acyl-ghrelin treatment leads to weight gain with low inflammation, high oxidative capacity and normal triglycerides in rat muscle - Barazzoni_2011_PLoS.One_6_e26224 |
| Author(s) : Barazzoni R , Zanetti M , Semolic A , Cattin MR , Pirulli A , Cattin L , Guarnieri G |
| Ref : PLoS ONE , 6 :e26224 , 2011 |
| Abstract : Barazzoni_2011_PLoS.One_6_e26224 |
| ESTHER : Barazzoni_2011_PLoS.One_6_e26224 |
| PubMedSearch : Barazzoni_2011_PLoS.One_6_e26224 |
| PubMedID: 22039445 |
| Title : Ghrelin octanoylation mediated by an orphan lipid transferase - Gutierrez_2008_Proc.Natl.Acad.Sci.U.S.A_105_6320 |
| Author(s) : Gutierrez JA , Solenberg PJ , Perkins DR , Willency JA , Knierman MD , Jin Z , Witcher DR , Luo S , Onyia JE , Hale JE |
| Ref : Proc Natl Acad Sci U S A , 105 :6320 , 2008 |
| Abstract : Gutierrez_2008_Proc.Natl.Acad.Sci.U.S.A_105_6320 |
| ESTHER : Gutierrez_2008_Proc.Natl.Acad.Sci.U.S.A_105_6320 |
| PubMedSearch : Gutierrez_2008_Proc.Natl.Acad.Sci.U.S.A_105_6320 |
| PubMedID: 18443287 |
| Title : The butyrylcholinesterase knockout mouse is obese on a high-fat diet - Li_2008_Chem.Biol.Interact_175_88 |
| Author(s) : Li B , Duysen EG , Lockridge O |
| Ref : Chemico-Biological Interactions , 175 :88 , 2008 |
| Abstract : Li_2008_Chem.Biol.Interact_175_88 |
| ESTHER : Li_2008_Chem.Biol.Interact_175_88 |
| PubMedSearch : Li_2008_Chem.Biol.Interact_175_88 |
| PubMedID: 18452903 |
| Title : A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India - Manoharan_2007_Clin.Chim.Acta_378_128 |
| Author(s) : Manoharan I , Boopathy R , Darvesh S , Lockridge O |
| Ref : Clinica Chimica Acta , 378 :128 , 2007 |
| Abstract : Manoharan_2007_Clin.Chim.Acta_378_128 |
| ESTHER : Manoharan_2007_Clin.Chim.Acta_378_128 |
| PubMedSearch : Manoharan_2007_Clin.Chim.Acta_378_128 |
| PubMedID: 17182021 |
| Title : Ghrelin interacts with human plasma lipoproteins - De Vriese_2007_Endocrinology_148_2355 |
| Author(s) : De Vriese C , Hacquebard M , Gregoire F , Carpentier Y , Delporte C |
| Ref : Endocrinology , 148 :2355 , 2007 |
| Abstract : De Vriese_2007_Endocrinology_148_2355 |
| ESTHER : De Vriese_2007_Endocrinology_148_2355 |
| PubMedSearch : De Vriese_2007_Endocrinology_148_2355 |
| PubMedID: 17289852 |
| Title : Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites - De Vriese_2004_Endocrinology_145_4997 |
| Author(s) : De Vriese C , Gregoire F , Lema-Kisoka R , Waelbroeck M , Robberecht P , Delporte C |
| Ref : Endocrinology , 145 :4997 , 2004 |
| Abstract : De Vriese_2004_Endocrinology_145_4997 |
| ESTHER : De Vriese_2004_Endocrinology_145_4997 |
| PubMedSearch : De Vriese_2004_Endocrinology_145_4997 |
| PubMedID: 15256494 |