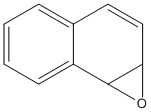
Naphthalene-1,2-epoxide
General
Type : Epoxide || Naphtyl ester || Naphthalen
Chemical_Nomenclature : 1a,7b-dihydronaphtho[1,2-b]oxirene
Canonical SMILES : C1=CC=C2C3C(O3)C=CC2=C1
InChI : InChI=1S\/C10H8O\/c1-2-4-8-7(3-1)5-6-9-10(8)11-9\/h1-6,9-10H
InChIKey : XQIJIALOJPIKGX-UHFFFAOYSA-N
Other name(s) : Naphthalene 1,2-oxide, 1a,7b-Dihydronaphth(1,2-b)oxirene, 1a,7b-dihydronaphtho[1,2-b]oxirene, SCHEMBL50866, CHEBI:52431
Target
Families : Epoxide_hydrolase
References (3)
| Title : Interindividual and interspecies variation in hepatic microsomal epoxide hydrolase activity: studies with cis-stilbene oxide, carbamazepine 10, 11-epoxide and naphthalene - Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| Author(s) : Kitteringham NR , Davis C , Howard N , Pirmohamed M , Park BK |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 278 :1018 , 1996 |
| Abstract : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| ESTHER : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| PubMedSearch : Kitteringham_1996_J.Pharmacol.Exp.Ther_278_1018 |
| PubMedID: 8819481 |
| Title : Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme - Wang_1982_Biochemistry_21_5769 |
| Author(s) : Wang P , Meijer J , Guengerich FP |
| Ref : Biochemistry , 21 :5769 , 1982 |
| Abstract : Wang_1982_Biochemistry_21_5769 |
| ESTHER : Wang_1982_Biochemistry_21_5769 |
| PubMedSearch : Wang_1982_Biochemistry_21_5769 |
| PubMedID: 6185139 |
| Gene_locus related to this paper: human-EPHX1 , human-EPHX2 |
| Title : Hydration of arene and alkene oxides by epoxide hydrase in human liver microsomes - Kapitulnik_1977_Clin.Pharmacol.Ther_21_158 |
| Author(s) : Kapitulnik J , Levin W , Morecki R , Dansette PM , Jerina DM , Conney AH |
| Ref : Clinical Pharmacology & Therapeutics , 21 :158 , 1977 |
| Abstract : Kapitulnik_1977_Clin.Pharmacol.Ther_21_158 |
| ESTHER : Kapitulnik_1977_Clin.Pharmacol.Ther_21_158 |
| PubMedSearch : Kapitulnik_1977_Clin.Pharmacol.Ther_21_158 |
| PubMedID: 65238 |