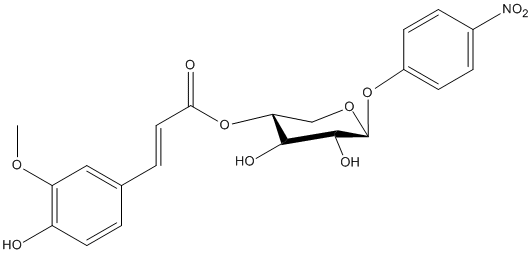
NPh-4-Fe-Xylp
General
Type : Glycoside || Feruloyl || pNP
Chemical_Nomenclature : [(3R,4R,5R,6S)-4,5-dihydroxy-6-(4-nitrophenoxy)oxan-3-yl] 3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate
Canonical SMILES : COC1=C(C=CC(=C1)C=CC(=O)OC2COC(C(C2O)O)OC3=CC=C(C=C3)[N+](=O)[O-])O
InChI : InChI=1S\/C21H21NO10\/c1-29-16-10-12(2-8-15(16)23)3-9-18(24)32-17-11-30-21(20(26)19(17)25)31-14-6-4-13(5-7-14)22(27)28\/h2-10,17,19-21,23,25-26H,11H2,1H3\/t17-,19+,20-,21+\/m1\/s1
InChIKey : RYZRSSQOQHWGBA-PMXSJFBMSA-N
Other name(s) : 4-Nitrophenyl 4-O-[(E)-3-methoxy-4-hydroxycinnamoyl]-beta-D-xylopyranoside, p-Nitrophenyl 4-O-[3-(3-methoxy-4-hydroxyphenyl)acryloyl]-beta-D-xylopyranoside
MW : 447.4
Formula : C21H21NO10
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : Esterase_phb
References (3)
| Title : Purification and characterization of a type B feruloyl esterase (StFAE-A) from the thermophilic fungus Sporotrichum thermophile - Topakas_2004_Appl.Microbiol.Biotechnol_63_686 |
| Author(s) : Topakas E , Stamatis H , Biely P , Christakopoulos P |
| Ref : Applied Microbiology & Biotechnology , 63 :686 , 2004 |
| Abstract : Topakas_2004_Appl.Microbiol.Biotechnol_63_686 |
| ESTHER : Topakas_2004_Appl.Microbiol.Biotechnol_63_686 |
| PubMedSearch : Topakas_2004_Appl.Microbiol.Biotechnol_63_686 |
| PubMedID: 14615854 |
| Gene_locus related to this paper: myctt-faeb |
| Title : Purification and properties of a feruloyl esterase involved in lignocellulose degradation by Aureobasidium pullulans - Rumbold_2003_Appl.Environ.Microbiol_69_5622 |
| Author(s) : Rumbold K , Biely P , Mastihubova M , Gudelj M , Gubitz G , Robra KH , Prior BA |
| Ref : Applied Environmental Microbiology , 69 :5622 , 2003 |
| Abstract : Rumbold_2003_Appl.Environ.Microbiol_69_5622 |
| ESTHER : Rumbold_2003_Appl.Environ.Microbiol_69_5622 |
| PubMedSearch : Rumbold_2003_Appl.Environ.Microbiol_69_5622 |
| PubMedID: 12957952 |
| Title : Differentiation of feruloyl esterases on synthetic substrates in alpha-arabinofuranosidase-coupled and ultraviolet-spectrophotometric assays - Biely_2002_Anal.Biochem_311_68 |
| Author(s) : Biely P , Mastihubova M , van Zyl WH , Prior BA |
| Ref : Analytical Biochemistry , 311 :68 , 2002 |
| Abstract : Biely_2002_Anal.Biochem_311_68 |
| ESTHER : Biely_2002_Anal.Biochem_311_68 |
| PubMedSearch : Biely_2002_Anal.Biochem_311_68 |
| PubMedID: 12441154 |