Myristoylcholine
General
Type : Trimethylammonium || Choline ester
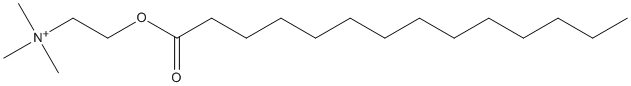
Chemical_Nomenclature : trimethyl(2-tetradecanoyloxyethyl)azanium
Canonical SMILES : CCCCCCCCCCCCCC(=O)OCC[N+](C)(C)C || CCCCCCCCCCCCCC(=O)OCC[N+](C)(C)C.[I-] || CCCCCCCCCCCCCC(=O)OCC[N+](C)(C)C.[Br-] || CCCCCCCCCCCCCC(=O)OCC[N+](C)(C)C.[Cl-]
InChI : InChI=1S\/C19H40NO2\/c1-5-6-7-8-9-10-11-12-13-14-15-16-19(21)22-18-17-20(2,3)4\/h5-18H2,1-4H3\/q+1 || InChI=1S\/C19H40NO2.HI\/c1-5-6-7-8-9-10-11-12-13-14-15-16-19(21)22-18-17-20(2,3)4\;\/h5-18H2,1-4H3\;1H\/q+1\;\/p-1 || InChI=1S\/C19H40NO2.BrH\/c1-5-6-7-8-9-10-11-12-13-14-15-16-19(21)22-18-17-20(2,3)4\;\/h5-18H2,1-4H3\;1H\/q+1\;\/p-1 || InChI=1S\/C19H40NO2.ClH\/c1-5-6-7-8-9-10-11-12-13-14-15-16-19(21)22-18-17-20(2,3)4\;\/h5-18H2,1-4H3\;1H\/q+1\;\/p-1
InChIKey : HYPHVSJUVHDHIL-UHFFFAOYSA-N || GAVXBOJISDNUPP-UHFFFAOYSA-M || GYVAQLUBQVAKEI-UHFFFAOYSA-M || CNJMYRYCYNAIOF-UHFFFAOYSA-M
Other name(s) : n,n,n-trimethyl-2-(tetradecanoyloxy)ethanaminium, SCHEMBL200400, ZINC60292359, Myristoylcholine bromide, Myristoyl choline, Myristoylcholine chloride
MW : 314.5
Formula : C19H40NO2+
CAS_number : 23464-67-7 || 108418-28-6 || 4277-89-8
PubChem :
UniChem :
Iuphar :
Target
Families : No family
References (4)
| Title : Cholinesterase-responsive supramolecular vesicle - Guo_2012_J.Am.Chem.Soc_134_10244 |
| Author(s) : Guo DS , Wang K , Wang YX , Liu Y |
| Ref : Journal of the American Chemical Society , 134 :10244 , 2012 |
| Abstract : Guo_2012_J.Am.Chem.Soc_134_10244 |
| ESTHER : Guo_2012_J.Am.Chem.Soc_134_10244 |
| PubMedSearch : Guo_2012_J.Am.Chem.Soc_134_10244 |
| PubMedID: 22686862 |
| Title : Acetylcholinesterase responsive polymeric supra-amphiphiles for controlled self-assembly and disassembly - Xing_2012_Langmuir_28_6032 |
| Author(s) : Xing Y , Wang C , Han P , Wang Z , Zhang X |
| Ref : Langmuir , 28 :6032 , 2012 |
| Abstract : Xing_2012_Langmuir_28_6032 |
| ESTHER : Xing_2012_Langmuir_28_6032 |
| PubMedSearch : Xing_2012_Langmuir_28_6032 |
| PubMedID: 22404254 |
| Title : Colorimetric assays for acetylcholinesterase activity and inhibitor screening based on the disassembly-assembly of a water-soluble polythiophene derivative - Li_2011_ACS.Appl.Mater.Interfaces_3_1306 |
| Author(s) : Li Y , Bai H , Li C , Shi G |
| Ref : ACS Appl Mater Interfaces , 3 :1306 , 2011 |
| Abstract : Li_2011_ACS.Appl.Mater.Interfaces_3_1306 |
| ESTHER : Li_2011_ACS.Appl.Mater.Interfaces_3_1306 |
| PubMedSearch : Li_2011_ACS.Appl.Mater.Interfaces_3_1306 |
| PubMedID: 21438627 |
| Title : Convenient and continuous fluorometric assay method for acetylcholinesterase and inhibitor screening based on the aggregation-induced emission - Wang_2009_Anal.Chem_81_4444 |
| Author(s) : Wang M , Gu X , Zhang G , Zhang D , Zhu D |
| Ref : Analytical Chemistry , 81 :4444 , 2009 |
| Abstract : Wang_2009_Anal.Chem_81_4444 |
| ESTHER : Wang_2009_Anal.Chem_81_4444 |
| PubMedSearch : Wang_2009_Anal.Chem_81_4444 |
| PubMedID: 19374428 |