Mycotoxin-T-2
General
Type : Natural || Toxin || Mycotoxin-metabolite
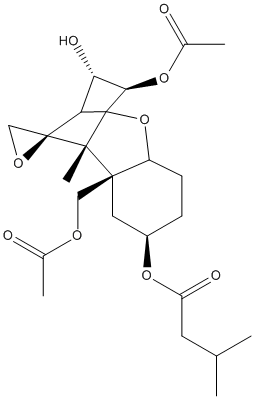
Chemical_Nomenclature : [(1S,2R,4S,7R,9R,10R,11S,12S)-11-acetyloxy-2-(acetyloxymethyl)-10-hydroxy-1,5-dimethylspiro[8-oxatricyclo[7.2.1.02,7]dodec-5-ene-12,2'-oxirane]-4-yl] 3-methylbutanoate
Canonical SMILES : CC1=CC2C(CC1OC(=O)CC(C)C)(C3(C(C(C(C34CO4)O2)O)OC(=O)C)C)COC(=O)C
InChI : InChI=1S\/C24H34O9\/c1-12(2)7-18(27)32-16-9-23(10-29-14(4)25)17(8-13(16)3)33-21-19(28)20(31-15(5)26)22(23,6)24(21)11-30-24\/h8,12,16-17,19-21,28H,7,9-11H2,1-6H3\/t16-,17+,19+,20+,21+,22+,23+,24-\/m0\/s1
InChIKey : BXFOFFBJRFZBQZ-QYWOHJEZSA-N
Other name(s) : T2 Toxin, T-2 Toxin, Insariotoxin, Isaritoxin, T-2, T-2 mycotoxin, mycotoxin T-2, CHEBI:9381, CHEMBL152423, SCHEMBL7536392
Target
Families : Carb_B_Chordata, Hormone-sensitive_lipase_like
References (18)
| Title : Thermophilic Carboxylesterases from Hydrothermal Vents of the Volcanic Island of Ischia Active on Synthetic and Biobased Polymers and Mycotoxins - Distaso_2023_Appl.Environ.Microbiol__e0170422 |
| Author(s) : Distaso MA , Chernikova TN , Bargiela R , Coscolin C , Stogios P , Gonzalez-Alfonso JL , Lemak S , Khusnutdinova AN , Plou FJ , Evdokimova E , Savchenko A , Lunev EA , Yakimov MM , Golyshina OV , Ferrer M , Yakunin AF , Golyshin PN |
| Ref : Applied Environmental Microbiology , :e0170422 , 2023 |
| Abstract : Distaso_2023_Appl.Environ.Microbiol__e0170422 |
| ESTHER : Distaso_2023_Appl.Environ.Microbiol__e0170422 |
| PubMedSearch : Distaso_2023_Appl.Environ.Microbiol__e0170422 |
| PubMedID: 36719236 |
| Gene_locus related to this paper: 9bact-estC55.8n1 , 9bact-IS10 |
| Title : Crystal structure of a family VIII beta-lactamase fold hydrolase reveals the molecular mechanism for its broad substrate scope - Cea-Rama_2022_FEBS.J__ |
| Author(s) : Cea-Rama I , Coscolin C , Gonzalez-Alfonso JL , Raj J , Vasiljevic M , Plou FJ , Ferrer M , Sanz-Aparicio J |
| Ref : Febs J , : , 2022 |
| Abstract : Cea-Rama_2022_FEBS.J__ |
| ESTHER : Cea-Rama_2022_FEBS.J__ |
| PubMedSearch : Cea-Rama_2022_FEBS.J__ |
| PubMedID: 35694902 |
| Title : Fluorine impairs carboxylesterase 1-mediated hydrolysis of T-2 toxin and increases its chondrocyte toxicity - Jia_2022_Front.Nutr_9_935112 |
| Author(s) : Jia Y , Shi S , Cheng B , Cheng S , Liu L , Meng P , Yang X , Chu X , Wen Y , Zhang F , Guo X |
| Ref : Front Nutr , 9 :935112 , 2022 |
| Abstract : Jia_2022_Front.Nutr_9_935112 |
| ESTHER : Jia_2022_Front.Nutr_9_935112 |
| PubMedSearch : Jia_2022_Front.Nutr_9_935112 |
| PubMedID: 35990316 |
| Title : An update on T-2 toxin and its modified forms: metabolism, immunotoxicity mechanism, and human exposure assessment - Wu_2020_Arch.Toxicol_94_3645 |
| Author(s) : Wu Q , Qin Z , Kuca K , You L , Zhao Y , Liu A , Musilek K , Chrienova Z , Nepovimova E , Oleksak P , Wu W , Wang X |
| Ref : Archives of Toxicology , 94 :3645 , 2020 |
| Abstract : Wu_2020_Arch.Toxicol_94_3645 |
| ESTHER : Wu_2020_Arch.Toxicol_94_3645 |
| PubMedSearch : Wu_2020_Arch.Toxicol_94_3645 |
| PubMedID: 32910237 |
| Title : Biotransformation enzyme activities and phase I metabolites analysis in Litopenaeus vannamei following intramuscular administration of T-2 toxin - Deng_2017_Drug.Chem.Toxicol__1 |
| Author(s) : Deng Y , Wang Y , Sun L , Lu P , Wang R , Ye L , Xu D , Ye R , Liu Y , Bi S , Gooneratne R |
| Ref : Drug & Chemical Toxicology , :1 , 2017 |
| Abstract : Deng_2017_Drug.Chem.Toxicol__1 |
| ESTHER : Deng_2017_Drug.Chem.Toxicol__1 |
| PubMedSearch : Deng_2017_Drug.Chem.Toxicol__1 |
| PubMedID: 28482697 |
| Title : The roles of carboxylesterase and CYP isozymes on the in vitro metabolism of T-2 toxin - Lin_2015_Mil.Med.Res_2_13 |
| Author(s) : Lin NN , Chen J , Xu B , Wei X , Guo L , Xie JW |
| Ref : Mil Med Res , 2 :13 , 2015 |
| Abstract : Lin_2015_Mil.Med.Res_2_13 |
| ESTHER : Lin_2015_Mil.Med.Res_2_13 |
| PubMedSearch : Lin_2015_Mil.Med.Res_2_13 |
| PubMedID: 26140218 |
| Title : Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: an update - Wu_2014_Arch.Toxicol_88_1309 |
| Author(s) : Wu QH , Wang X , Yang W , Nussler AK , Xiong LY , Kuca K , Dohnal V , Zhang XJ , Yuan ZH |
| Ref : Archives of Toxicology , 88 :1309 , 2014 |
| Abstract : Wu_2014_Arch.Toxicol_88_1309 |
| ESTHER : Wu_2014_Arch.Toxicol_88_1309 |
| PubMedSearch : Wu_2014_Arch.Toxicol_88_1309 |
| PubMedID: 24894432 |
| Title : Glucosylation and other biotransformations of T-2 toxin by yeasts of the trichomonascus clade - McCormick_2012_Appl.Environ.Microbiol_78_8694 |
| Author(s) : McCormick SP , Price NP , Kurtzman CP |
| Ref : Applied Environmental Microbiology , 78 :8694 , 2012 |
| Abstract : McCormick_2012_Appl.Environ.Microbiol_78_8694 |
| ESTHER : McCormick_2012_Appl.Environ.Microbiol_78_8694 |
| PubMedSearch : McCormick_2012_Appl.Environ.Microbiol_78_8694 |
| PubMedID: 23042183 |
| Title : Integrated transcriptional and proteomic analysis with in vitro biochemical assay reveal the important role of CYP3A46 in T-2 toxin hydroxylation in porcine primary hepatocytes - Wang_2011_Mol.Cell.Proteomics_10_M111 008748 |
| Author(s) : Wang J , Jiang J , Zhang H , Cai H , Li C , Li K , Liu J , Guo X , Zou G , Wang D , Deng Y , Dai J |
| Ref : Mol Cell Proteomics , 10 :M111 008748 , 2011 |
| Abstract : Wang_2011_Mol.Cell.Proteomics_10_M111 008748 |
| ESTHER : Wang_2011_Mol.Cell.Proteomics_10_M111 008748 |
| PubMedSearch : Wang_2011_Mol.Cell.Proteomics_10_M111 008748 |
| PubMedID: 21685020 |
| Title : A comparison of hepatic in vitro metabolism of T-2 toxin in rats, pigs, chickens, and carp - Wu_2011_Xenobiotica_41_863 |
| Author(s) : Wu Q , Huang L , Liu Z , Yao M , Wang Y , Dai M , Yuan Z |
| Ref : Xenobiotica , 41 :863 , 2011 |
| Abstract : Wu_2011_Xenobiotica_41_863 |
| ESTHER : Wu_2011_Xenobiotica_41_863 |
| PubMedSearch : Wu_2011_Xenobiotica_41_863 |
| PubMedID: 21745144 |
| Title : Degradation of fumonisin B1 by the consecutive action of two bacterial enzymes - Heinl_2010_J.Biotechnol_145_120 |
| Author(s) : Heinl S , Hartinger D , Thamhesl M , Vekiru E , Krska R , Schatzmayr G , Moll WD , Grabherr R |
| Ref : J Biotechnol , 145 :120 , 2010 |
| Abstract : Heinl_2010_J.Biotechnol_145_120 |
| ESTHER : Heinl_2010_J.Biotechnol_145_120 |
| PubMedSearch : Heinl_2010_J.Biotechnol_145_120 |
| PubMedID: 19922747 |
| Gene_locus related to this paper: sphmc-FumD |
| Title : Effect of nutritional indoles on activity of xenobiotic metabolism enzymes and T-2 toxicity in rats - Kravchenko_2001_Bull.Exp.Biol.Med_131_544 |
| Author(s) : Kravchenko LV , Avren'eva LI , Guseva GV , Posdnyakov AL , Tutel'yan VA |
| Ref : Bulletin of Experimental Biology & Medicine , 131 :544 , 2001 |
| Abstract : Kravchenko_2001_Bull.Exp.Biol.Med_131_544 |
| ESTHER : Kravchenko_2001_Bull.Exp.Biol.Med_131_544 |
| PubMedSearch : Kravchenko_2001_Bull.Exp.Biol.Med_131_544 |
| PubMedID: 11586402 |
| Title : Partial purification and characterization of an esterase from Fusarium sporotrichioides - Park_1996_Nat.Toxins_4_108 |
| Author(s) : Park JJ , Chu FS |
| Ref : Nat Toxins , 4 :108 , 1996 |
| Abstract : Park_1996_Nat.Toxins_4_108 |
| ESTHER : Park_1996_Nat.Toxins_4_108 |
| PubMedSearch : Park_1996_Nat.Toxins_4_108 |
| PubMedID: 8743931 |
| Title : Metabolism of T-2 toxin by rat liver carboxylesterase - Johnsen_1986_Biochem.Pharmacol_35_1469 |
| Author(s) : Johnsen H , Odden E , Lie O , Johnsen BA , Fonnum F |
| Ref : Biochemical Pharmacology , 35 :1469 , 1986 |
| Abstract : Johnsen_1986_Biochem.Pharmacol_35_1469 |
| ESTHER : Johnsen_1986_Biochem.Pharmacol_35_1469 |
| PubMedSearch : Johnsen_1986_Biochem.Pharmacol_35_1469 |
| PubMedID: 3707611 |
| Title : Biochemical changes in subacute mycotoxicosis induced by T-2 toxin in rats - Kravchenko_1986_Toxicology_42_77 |
| Author(s) : Kravchenko LV , Tutelyan VA , Vasilyev AV , Kranauskas AE , Avrenyeva LI |
| Ref : Toxicology , 42 :77 , 1986 |
| Abstract : Kravchenko_1986_Toxicology_42_77 |
| ESTHER : Kravchenko_1986_Toxicology_42_77 |
| PubMedSearch : Kravchenko_1986_Toxicology_42_77 |
| PubMedID: 3798460 |
| Title : Carboxylesterases, importance for detoxification of organophosphorus anticholinesterases and trichothecenes - Fonnum_1985_Fundam.Appl.Toxicol_5_S29 |
| Author(s) : Fonnum F , Sterri SH , Aas P , Johnsen H |
| Ref : Fundamental & Applied Toxicology , 5 :S29 , 1985 |
| Abstract : Fonnum_1985_Fundam.Appl.Toxicol_5_S29 |
| ESTHER : Fonnum_1985_Fundam.Appl.Toxicol_5_S29 |
| PubMedSearch : Fonnum_1985_Fundam.Appl.Toxicol_5_S29 |
| PubMedID: 4092894 |
| Title : Metabolism of trichothecene mycotoxins. II. Substrate specificity of microsomal deacetylation of trichothecenes - Ohta_1978_J.Biochem_84_697 |
| Author(s) : Ohta M , Matsumoto H , Ishii K , Ueno Y |
| Ref : J Biochem , 84 :697 , 1978 |
| Abstract : Ohta_1978_J.Biochem_84_697 |
| ESTHER : Ohta_1978_J.Biochem_84_697 |
| PubMedSearch : Ohta_1978_J.Biochem_84_697 |
| PubMedID: 721800 |
| Title : Metabolism of trichothecene mycotoxins. I. Microsomal deacetylation of T-2 toxin in animal tissues - Ohta_1977_J.Biochem_82_1591 |
| Author(s) : Ohta M , Ishii K , Ueno Y |
| Ref : J Biochem , 82 :1591 , 1977 |
| Abstract : Ohta_1977_J.Biochem_82_1591 |
| ESTHER : Ohta_1977_J.Biochem_82_1591 |
| PubMedSearch : Ohta_1977_J.Biochem_82_1591 |
| PubMedID: 599145 |