Monostearin
General
Type : Acyl-Glycerol || Monoacylglycerol || Fatty acid ester || Water-insoluble medium and long chain ester || Stearate
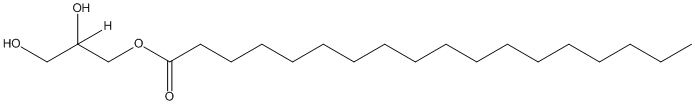
Chemical_Nomenclature : 2,3-dihydroxypropyl octadecanoate
Canonical SMILES : CCCCCCCCCCCCCCCCCC(=O)OCC(CO)O
InChI : InChI=1S\/C21H42O4\/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-21(24)25-19-20(23)18-22\/h20,22-23H,2-19H2,1H3
InChIKey : VBICKXHEKHSIBG-UHFFFAOYSA-N
Other name(s) : Glyceryl monostearate, Glyceryl stearate, 1-Stearoyl-rac-glycerol, Tegin
Target
References (3)
| Title : Discovery of a non-canonical prototype long-chain monoacylglycerol lipase through a structure-based endogenous reaction intermediate complex - Pinotsis_2023_Nat.Commun_14_7649 |
| Author(s) : Pinotsis N , Kruger A , Tomas N , Chatziefthymiou SD , Litz C , Mortensen SA , Daffe M , Marrakchi H , Antranikian G , Wilmanns M |
| Ref : Nat Commun , 14 :7649 , 2023 |
| Abstract : Pinotsis_2023_Nat.Commun_14_7649 |
| ESTHER : Pinotsis_2023_Nat.Commun_14_7649 |
| PubMedSearch : Pinotsis_2023_Nat.Commun_14_7649 |
| PubMedID: 38012138 |
| Title : Carboxylesterase 2 proteins are efficient diglyceride and monoglyceride lipases possibly implicated in metabolic disease - Chalhoub_2021_J.Lipid.Res__100075 |
| Author(s) : Chalhoub G , Kolleritsch S , Maresch LK , Taschler U , Pajed L , Tilp A , Natmessnig H , Rosina P , Kien B , Radner FPW , Schicho R , Oberer M , Schoiswohl G , Haemmerle G |
| Ref : J Lipid Res , :100075 , 2021 |
| Abstract : Chalhoub_2021_J.Lipid.Res__100075 |
| ESTHER : Chalhoub_2021_J.Lipid.Res__100075 |
| PubMedSearch : Chalhoub_2021_J.Lipid.Res__100075 |
| PubMedID: 33872605 |
| Gene_locus related to this paper: human-CES2 , mouse-Ces2a , mouse-Ces2b , mouse-Ces2c |
| Title : Solvent-free enzymatic transesterification of ethyl ferulate and monostearin: optimized by response surface methodology - Sun_2012_J.Biotechnol_164_340 |
| Author(s) : Sun S , Song F , Bi Y , Yang G , Liu W |
| Ref : J Biotechnol , 164 :340 , 2012 |
| Abstract : Sun_2012_J.Biotechnol_164_340 |
| ESTHER : Sun_2012_J.Biotechnol_164_340 |
| PubMedSearch : Sun_2012_J.Biotechnol_164_340 |
| PubMedID: 23376620 |