Mivacurium
General
Type : Neuromuscular Nondepolarizing Agents || Drug || Isoquinoline || Bisquaternary || Not A\/B H target
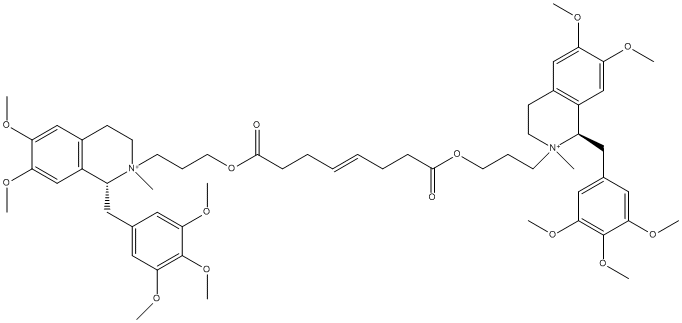
Chemical_Nomenclature : bis[3-[(1S)-6,7-dimethoxy-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]-3,4-dihydro-1H-isoquinolin-2-yl]propyl] oct-4-enedioate dichloride
Canonical SMILES : C[N+]1(CCC2=CC(=C(C=C2C1CC3=CC(=C(C(=C3)OC)OC)OC)OC)OC)CCCOC(=O)CCC=CCCC(=O)OCCC[N+]4(CCC5=CC(=C(C=C5C4CC6=CC(=C(C(=C6)OC)OC)OC)OC)OC)C
InChI : InChI=1S\/C58H80N2O14\/c1-59(25-21-41-35-47(63-3)49(65-5)37-43(41)45(59)29-39-31-51(67-7)57(71-11)52(32-39)68-8)23-17-27-73-55(61)19-15-13-14-16-20-56(62)74-28-18-24-60(2)26-22-42-36-48(64-4)50(66-6)38-44(42)46(60)30-40-33-53(69-9)58(72-12)54(34-40)70-10\/h13-14,31-38,45-46H,15-30H2,1-12H3\/q+2\/b14-13+\/t45-,46-,59?,60?\/m1\/s1
InChIKey : ILVYCEVXHALBSC-OTBYEXOQSA-N
Other name(s) : BW B1090U Mivacron, Mivacron, BW B1090U
MW : 1029.26
Formula : C58H80N2O14
CAS_number :
PubChem :
UniChem :
Iuphar :
Target
Families : BCHE
References (21)
| Title : Cholinesterase inhibition by potato glycoalkaloids slows mivacurium metabolism - McGehee_2000_Anesthesiology_93_510 |
| Author(s) : McGehee DS , Krasowski MD , Fung DL , Wilson B , Gronert GA , Moss J |
| Ref : Anesthesiology , 93 :510 , 2000 |
| Abstract : McGehee_2000_Anesthesiology_93_510 |
| ESTHER : McGehee_2000_Anesthesiology_93_510 |
| PubMedSearch : McGehee_2000_Anesthesiology_93_510 |
| PubMedID: 10910502 |
| Title : Neuromuscular interactions between mivacurium and esmolol in rabbits - Kim_1998_Anaesthesia_53_140 |
| Author(s) : Kim KS , Kim KH , Shin WJ , Yoo HK |
| Ref : Anaesthesia , 53 :140 , 1998 |
| Abstract : Kim_1998_Anaesthesia_53_140 |
| ESTHER : Kim_1998_Anaesthesia_53_140 |
| PubMedSearch : Kim_1998_Anaesthesia_53_140 |
| PubMedID: 9534636 |
| Title : [Muscle relaxants. New substances and neuromuscular monitoring] - Diefenbach_1997_Anaesthesist_46_3 |
| Author(s) : Diefenbach C , Nigrovic V , Mellinghoff H , Buzello W |
| Ref : Anaesthesist , 46 :3 , 1997 |
| Abstract : Diefenbach_1997_Anaesthesist_46_3 |
| ESTHER : Diefenbach_1997_Anaesthesist_46_3 |
| PubMedSearch : Diefenbach_1997_Anaesthesist_46_3 |
| PubMedID: 9082865 |
| Title : [The clinical pharmacology of mivacurium] - Diefenbach_1997_Anaesthesist_46_385 |
| Author(s) : Diefenbach C , Mellinghoff H |
| Ref : Anaesthesist , 46 :385 , 1997 |
| Abstract : Diefenbach_1997_Anaesthesist_46_385 |
| ESTHER : Diefenbach_1997_Anaesthesist_46_385 |
| PubMedSearch : Diefenbach_1997_Anaesthesist_46_385 |
| PubMedID: 9245207 |
| Title : [Esters and stereoisomers] - Nigrovic_1997_Anaesthesist_46_282 |
| Author(s) : Nigrovic V , Diefenbach C , Mellinghoff H |
| Ref : Anaesthesist , 46 :282 , 1997 |
| Abstract : Nigrovic_1997_Anaesthesist_46_282 |
| ESTHER : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedSearch : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedID: 9229981 |
| Title : Plasma cholinesterase deficiency. - Dell_1996_J.Perianesth.Nurs_11_304 |
| Author(s) : Dell DD , Kehoe C |
| Ref : Journal of Perianesth Nurs , 11 :304 , 1996 |
| Abstract : Dell_1996_J.Perianesth.Nurs_11_304 |
| ESTHER : Dell_1996_J.Perianesth.Nurs_11_304 |
| PubMedSearch : Dell_1996_J.Perianesth.Nurs_11_304 |
| PubMedID: 8970294 |
| Title : Pseudocholinesterase-mediated hydrolysis is superior to neostigmine for reversal of mivacurium-induced paralysis in vitro - Yang_1996_Anesthesiology_84_936 |
| Author(s) : Yang HS , Goudsouzian N , Martyn JA |
| Ref : Anesthesiology , 84 :936 , 1996 |
| Abstract : Yang_1996_Anesthesiology_84_936 |
| ESTHER : Yang_1996_Anesthesiology_84_936 |
| PubMedSearch : Yang_1996_Anesthesiology_84_936 |
| PubMedID: 8638849 |
| Title : Mivacurium chloride--a comparative profile - Diffenbach_1995_Acta.Anaesthesiol.Scand.Suppl_106_23 |
| Author(s) : Diffenbach C , Buzello W , Mellinghoff H |
| Ref : Acta Anaesthesiologica Scandinavica Supplementum , 106 :23 , 1995 |
| Abstract : Diffenbach_1995_Acta.Anaesthesiol.Scand.Suppl_106_23 |
| ESTHER : Diffenbach_1995_Acta.Anaesthesiol.Scand.Suppl_106_23 |
| PubMedSearch : Diffenbach_1995_Acta.Anaesthesiol.Scand.Suppl_106_23 |
| PubMedID: 8533540 |
| Title : Mivacurium-induced prolonged neuromuscular block [see comments] - Sockalingam_1995_Br.J.Anaesth_74_234 |
| Author(s) : Sockalingam I , Green DW |
| Ref : British Journal of Anaesthesia , 74 :234 , 1995 |
| Abstract : Sockalingam_1995_Br.J.Anaesth_74_234 |
| ESTHER : Sockalingam_1995_Br.J.Anaesth_74_234 |
| PubMedSearch : Sockalingam_1995_Br.J.Anaesth_74_234 |
| PubMedID: 7696076 |
| Title : Prolonged neuromuscular block associated with mivacurium [see comments] - Fox_1995_Br.J.Anaesth_74_237 |
| Author(s) : Fox MH , Hunt PC |
| Ref : British Journal of Anaesthesia , 74 :237 , 1995 |
| Abstract : Fox_1995_Br.J.Anaesth_74_237 |
| ESTHER : Fox_1995_Br.J.Anaesth_74_237 |
| PubMedSearch : Fox_1995_Br.J.Anaesth_74_237 |
| PubMedID: 7696077 |
| Title : Comparison of the neuromuscular effects of mivacurium and suxamethonium in infants and children - Cook_1995_Acta.Anaesth.Scand.Supplementum_106_35 |
| Author(s) : Cook DR , Gronert BJ , Woelfel SK |
| Ref : Acta Anaesthesiologica Scandinavica Supplementum , 106 :35 , 1995 |
| Abstract : Cook_1995_Acta.Anaesth.Scand.Supplementum_106_35 |
| ESTHER : Cook_1995_Acta.Anaesth.Scand.Supplementum_106_35 |
| PubMedSearch : Cook_1995_Acta.Anaesth.Scand.Supplementum_106_35 |
| PubMedID: 8533543 |
| Title : Mivacurium in special patient groups. - Jones_1995_Acta.Anaesth.Scand.Supplementum_106_47 |
| Author(s) : Jones RM |
| Ref : Acta Anaesthesiologica Scandinavica Supplementum , 106 :47 , 1995 |
| Abstract : Jones_1995_Acta.Anaesth.Scand.Supplementum_106_47 |
| ESTHER : Jones_1995_Acta.Anaesth.Scand.Supplementum_106_47 |
| PubMedSearch : Jones_1995_Acta.Anaesth.Scand.Supplementum_106_47 |
| PubMedID: 8533546 |
| Title : Recovery of train-of-four after mivacurium - Brull_1995_Can.J.Anaesth_42_28 |
| Author(s) : Brull SJ , Connelly NR , Silverman DG |
| Ref : Canadian Journal of Anaesthesia , 42 :28 , 1995 |
| Abstract : Brull_1995_Can.J.Anaesth_42_28 |
| ESTHER : Brull_1995_Can.J.Anaesth_42_28 |
| PubMedSearch : Brull_1995_Can.J.Anaesth_42_28 |
| PubMedID: 7889581 |
| Title : Changes in plasma cholinesterase activity and mivacurium neuromuscular block in response to normothermic cardiopulmonary bypass - Diefenbach_1995_Anesth.Analg_80_1088 |
| Author(s) : Diefenbach C , Abel M , Rump AF , Grond S , Korb H , Buzello W |
| Ref : Anesthesia & Analgesia , 80 :1088 , 1995 |
| Abstract : Diefenbach_1995_Anesth.Analg_80_1088 |
| ESTHER : Diefenbach_1995_Anesth.Analg_80_1088 |
| PubMedSearch : Diefenbach_1995_Anesth.Analg_80_1088 |
| PubMedID: 7762834 |
| Title : Enzymatic antagonism of mivacurium-induced neuromuscular blockade by human plasma cholinesterase - Naguib_1995_Anesthesiology_83_694 |
| Author(s) : Naguib M , Daoud W , El-Gammal M , Ammar A , Turkistani A , Selim M , Altamimi W , Sohaibani MO |
| Ref : Anesthesiology , 83 :694 , 1995 |
| Abstract : Naguib_1995_Anesthesiology_83_694 |
| ESTHER : Naguib_1995_Anesthesiology_83_694 |
| PubMedSearch : Naguib_1995_Anesthesiology_83_694 |
| PubMedID: 7574048 |
| Title : Spontaneous recovery or evoked reversal of neuromuscular block - Mirakhur_1995_Acta.Anaesthesiol.Scand.Suppl_106_62 |
| Author(s) : Mirakhur RK |
| Ref : Acta Anaesthesiologica Scandinavica Supplementum , 106 :62 , 1995 |
| Abstract : Mirakhur_1995_Acta.Anaesthesiol.Scand.Suppl_106_62 |
| ESTHER : Mirakhur_1995_Acta.Anaesthesiol.Scand.Suppl_106_62 |
| PubMedSearch : Mirakhur_1995_Acta.Anaesthesiol.Scand.Suppl_106_62 |
| PubMedID: 8533549 |
| Title : Edrophonium increases mivacurium concentrations during constant mivacurium infusion, and large doses minimally antagonize paralysis [see comments] - Hart_1995_Anesthesiology_82_912 |
| Author(s) : Hart PS , Wright PM , Brown R , Lau M , Sharma ML , Miller RD , Gruenke L , Fisher DM |
| Ref : Anesthesiology , 82 :912 , 1995 |
| Abstract : Hart_1995_Anesthesiology_82_912 |
| ESTHER : Hart_1995_Anesthesiology_82_912 |
| PubMedSearch : Hart_1995_Anesthesiology_82_912 |
| PubMedID: 7717563 |
| Title : Mivacurium apnoea [letter\; comment] - |
| Author(s) : Lightman DJ |
| Ref : Anaesthesia , 49 :835 , 1994 |
| PubMedID: 7978163 |
| Title : Mivacurium. A review of its pharmacology and therapeutic potential in general anaesthesia - Frampton_1993_Drugs_45_1066 |
| Author(s) : Frampton JE , McTavish D |
| Ref : Drugs , 45 :1066 , 1993 |
| Abstract : Frampton_1993_Drugs_45_1066 |
| ESTHER : Frampton_1993_Drugs_45_1066 |
| PubMedSearch : Frampton_1993_Drugs_45_1066 |
| PubMedID: 7691494 |
| Title : Clinical pharmacology of mivacurium chloride: a review - Basta_1992_J.Clin.Anesth_4_153 |
| Author(s) : Basta SJ |
| Ref : Journal of Clinical Anesthesia , 4 :153 , 1992 |
| Abstract : Basta_1992_J.Clin.Anesth_4_153 |
| ESTHER : Basta_1992_J.Clin.Anesth_4_153 |
| PubMedSearch : Basta_1992_J.Clin.Anesth_4_153 |
| PubMedID: 1373287 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |