Methylprednisolone-acetate
General
Type : Acetate || Steroid || Phenanthrene || Drug
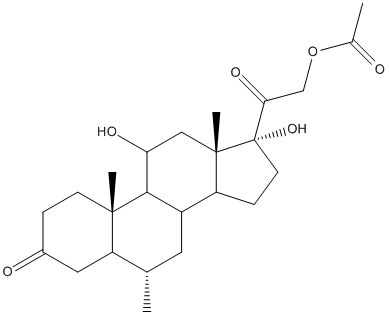
Chemical_Nomenclature : [2-[(6S,10R,11S,13S,17R)-11,17-dihydroxy-6,10,13-trimethyl-3-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate
Canonical SMILES : CC1CC2C3CCC(C3(CC(C2C4(C1=CC(=O)C=C4)C)O)C)(C(=O)COC(=O)C)O
InChI : InChI=1S\/C24H32O6\/c1-13-9-16-17-6-8-24(29,20(28)12-30-14(2)25)23(17,4)11-19(27)21(16)22(3)7-5-15(26)10-18(13)22\/h5,7,10,13,16-17,19,21,27,29H,6,8-9,11-12H2,1-4H3\/t13-,16?,17?,19-,21?,22-,23-,24-\/m0\/s1
InChIKey : PLBHSZGDDKCEHR-UFBGDBPHSA-N
Other name(s) : Depo-medrate, Medrol, Medrone, 11beta,17alpha,21-Trihydroxy-6alpha-methyl-1,4-pregnadiene-3,20-dione, SCHEMBL3437554
Target
Families : BCHE
References (2)
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Hydrolysis of methylprednisolone acetate by human serum cholinesterase - |
| Author(s) : Myers C , Lockridge O , La Du BN |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 10 :279 , 1982 |
| PubMedID: 6125364 |