Methyl-farnesoate
General
Type : Natural
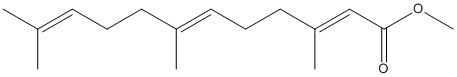
Chemical_Nomenclature : methyl (2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienoate
Canonical SMILES : CC(=CCCC(=CCCC(=CC(=O)OC)C)C)C
InChI : InChI=1S\/C16H26O2\/c1-13(2)8-6-9-14(3)10-7-11-15(4)12-16(17)18-5\/h8,10,12H,6-7,9,11H2,1-5H3\/b14-10+,15-12+
InChIKey : NWKXNIPBVLQYAB-VDQVFBMKSA-N
Other name(s) : Methyl farnesoate, Methyl (2E,6E)-farnesoate, (E,E)-Methyl farnesoate, (2E,6E)-farnesyl methyl ester
Target
Families : Juvenile_hormone_esterase, Carb_B_Arthropoda
References (7)
| Title : The miRNAs let-7b and miR-141 Coordinately Regulate Vitellogenesis by Modulating Methyl Farnesoate Degradation in the Swimming Crab Portunus trituberculatus - Yu_2023_Int.J.Mol.Sci_25_279 |
| Author(s) : Yu X , Zhang M , Liu P , Li J , Gao B , Meng X |
| Ref : Int J Mol Sci , 25 : , 2023 |
| Abstract : Yu_2023_Int.J.Mol.Sci_25_279 |
| ESTHER : Yu_2023_Int.J.Mol.Sci_25_279 |
| PubMedSearch : Yu_2023_Int.J.Mol.Sci_25_279 |
| PubMedID: 38203450 |
| Gene_locus related to this paper: portr-a0a1i9kj57 , portr-a0a1i9ky23 |
| Title : Identification, characterization and mRNA transcript abundance profiles of the carboxylesterase (CXE5) gene in Eriocheir sinensis suggest that it may play a role in methyl farnesoate degradation - Li_2021_Comp.Biochem.Physiol.B.Biochem.Mol.Biol__110630 |
| Author(s) : Li X , Chen T , Xu R , Huang M , Huang J , Xie Q , Liu F , Su S , Ma K |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , :110630 , 2021 |
| Abstract : Li_2021_Comp.Biochem.Physiol.B.Biochem.Mol.Biol__110630 |
| ESTHER : Li_2021_Comp.Biochem.Physiol.B.Biochem.Mol.Biol__110630 |
| PubMedSearch : Li_2021_Comp.Biochem.Physiol.B.Biochem.Mol.Biol__110630 |
| PubMedID: 34062270 |
| Gene_locus related to this paper: erisi-a0a7d5ly28 |
| Title : Molecular cloning and expression analysis of a prawn (Macrobrachium rosenbergii) juvenile hormone esterase-like carboxylesterase following immune challenge - Zhu_2018_Fish.Shellfish.Immunol_80_10 |
| Author(s) : Zhu XJ , Xiong Y , He W , Jin Y , Qian YQ , Liu J , Dai ZM |
| Ref : Fish Shellfish Immunol , 80 :10 , 2018 |
| Abstract : Zhu_2018_Fish.Shellfish.Immunol_80_10 |
| ESTHER : Zhu_2018_Fish.Shellfish.Immunol_80_10 |
| PubMedSearch : Zhu_2018_Fish.Shellfish.Immunol_80_10 |
| PubMedID: 29803663 |
| Title : Molecular cloning, characterization and expression analysis of two juvenile hormone esterase-like carboxylesterase cDNAs in Chinese mitten crab, Eriocheir sinensis - Xu_2017_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_205_46 |
| Author(s) : Xu Y , Zhao M , Deng Y , Yang Y , Li X , Lu Q , Ge J , Pan J , Xu Z |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 205 :46 , 2017 |
| Abstract : Xu_2017_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_205_46 |
| ESTHER : Xu_2017_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_205_46 |
| PubMedSearch : Xu_2017_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_205_46 |
| PubMedID: 28077333 |
| Gene_locus related to this paper: erisi-a0a1l5jht6 |
| Title : Cloning of two carboxylesterase cDNAs from the swimming crab Portunus trituberculatus: Molecular evidences for their putative roles in methyl farnesotae degradation - Tao_2016_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_203_100 |
| Author(s) : Tao T , Xie X , Liu M , Jiang Q , Zhu D |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 203 :100 , 2016 |
| Abstract : Tao_2016_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_203_100 |
| ESTHER : Tao_2016_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_203_100 |
| PubMedSearch : Tao_2016_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_203_100 |
| PubMedID: 27720728 |
| Gene_locus related to this paper: portr-a0a1i9kj57 , portr-a0a1i9ky23 |
| Title : Two juvenile hormone esterase-like carboxylesterase cDNAs from a Pandalus shrimp (Pandalopsis japonica): cloning, tissue expression, and effects of eyestalk ablation - Lee_2011_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_159_148 |
| Author(s) : Lee SO , Jeon JM , Oh CW , Kim YM , Kang CK , Lee DS , Mykles DL , Kim HW |
| Ref : Comparative Biochemistry & Physiology B Biochem Mol Biol , 159 :148 , 2011 |
| Abstract : Lee_2011_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_159_148 |
| ESTHER : Lee_2011_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_159_148 |
| PubMedSearch : Lee_2011_Comp.Biochem.Physiol.B.Biochem.Mol.Biol_159_148 |
| PubMedID: 21497668 |
| Gene_locus related to this paper: 9euca-f5a5q6 , 9euca-f5a5q7 |
| Title : Distribution and regulation of esterases that hydrolyze methyl farnesoate in Homarus americanus and other crustaceans - Homola_1997_Gen.Comp.Endocrinol_106_62 |
| Author(s) : Homola E , Chang ES |
| Ref : General & Comparative Endocrinology , 106 :62 , 1997 |
| Abstract : Homola_1997_Gen.Comp.Endocrinol_106_62 |
| ESTHER : Homola_1997_Gen.Comp.Endocrinol_106_62 |
| PubMedSearch : Homola_1997_Gen.Comp.Endocrinol_106_62 |
| PubMedID: 9126466 |