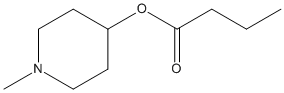
Methyl-4-piperidinyl-N-butyrate
General
Type : PET probe || Butyrate
Chemical_Nomenclature : (1-methylpiperidin-4-yl) butanoate
Canonical SMILES : CCCC(=O)OC1CCN(CC1)C
InChI : InChI=1S\/C10H19NO2\/c1-3-4-10(12)13-9-5-7-11(2)8-6-9\/h9H,3-8H2,1-2H3
InChIKey : ZDIPDZCEYMNVHW-UHFFFAOYSA-N
Other name(s) : (11)C-MP4B, 11C-MP4B, MP4B, 1-11C-methyl-4-piperidinyl n-butyrate, 1-Methylpiperidin-4-yl butanoate, methyl-4-piperidinyl butyrate, CHEMBL177357, SCHEMBL5844533
Target
Families : BCHE
References (9)
| Title : Biochemical and histochemical comparison of cholinesterases in normal and Alzheimer brain tissues - Darvesh_2010_Curr.Alzheimer.Res_7_386 |
| Author(s) : Darvesh S , Reid GA , Martin E |
| Ref : Curr Alzheimer Res , 7 :386 , 2010 |
| Abstract : Darvesh_2010_Curr.Alzheimer.Res_7_386 |
| ESTHER : Darvesh_2010_Curr.Alzheimer.Res_7_386 |
| PubMedSearch : Darvesh_2010_Curr.Alzheimer.Res_7_386 |
| PubMedID: 19939227 |
| Title : 1-11C-methyl-4-piperidinyl-N-butyrate radiation dosimetry in humans by dynamic organ-specific evaluation - Virta_2008_J.Nucl.Med_49_347 |
| Author(s) : Virta JR , Tolvanen T , Nagren K , Bruck A , Roivainen A , Rinne JO |
| Ref : J Nucl Med , 49 :347 , 2008 |
| Abstract : Virta_2008_J.Nucl.Med_49_347 |
| ESTHER : Virta_2008_J.Nucl.Med_49_347 |
| PubMedSearch : Virta_2008_J.Nucl.Med_49_347 |
| PubMedID: 18287260 |
| Title : Imaging butyrylcholinesterase activity in Alzheimer's disease - |
| Author(s) : Kuhl DE , Koeppe RA , Snyder SE , Minoshima S , Frey KA , Kilbourn MR |
| Ref : Annals of Neurology , 60 :746 , 2006 |
| PubMedID: 17192935 |
| Title : In vivo butyrylcholinesterase activity is not increased in Alzheimer's disease synapses - Kuhl_2006_Ann.Neurol_59_13 |
| Author(s) : Kuhl DE , Koeppe RA , Snyder SE , Minoshima S , Frey KA , Kilbourn MR |
| Ref : Annals of Neurology , 59 :13 , 2006 |
| Abstract : Kuhl_2006_Ann.Neurol_59_13 |
| ESTHER : Kuhl_2006_Ann.Neurol_59_13 |
| PubMedSearch : Kuhl_2006_Ann.Neurol_59_13 |
| PubMedID: 16278840 |
| Title : N-[18F]fluoroethylpiperidin-4-ylmethyl butyrate: a novel radiotracer for quantifying brain butyrylcholinesterase activity by positron emission tomography - Kikuchi_2004_Bioorg.Med.Chem.Lett_14_1927 |
| Author(s) : Kikuchi T , Zhang MR , Ikota N , Fukushi K , Okamura T , Suzuki K , Arano Y , Irie T |
| Ref : Bioorganic & Medicinal Chemistry Lett , 14 :1927 , 2004 |
| Abstract : Kikuchi_2004_Bioorg.Med.Chem.Lett_14_1927 |
| ESTHER : Kikuchi_2004_Bioorg.Med.Chem.Lett_14_1927 |
| PubMedSearch : Kikuchi_2004_Bioorg.Med.Chem.Lett_14_1927 |
| PubMedID: 15050629 |
| Title : Biodistribution and blood metabolism of 1-11C-methyl-4-piperidinyl n-butyrate in humans: an imaging agent for in vivo assessment of butyrylcholinesterase activity with PET - Roivainen_2004_J.Nucl.Med_45_2032 |
| Author(s) : Roivainen A , Rinne J , Virta J , Jarvenpaa T , Salomaki S , Yu M , Nagren K |
| Ref : J Nucl Med , 45 :2032 , 2004 |
| Abstract : Roivainen_2004_J.Nucl.Med_45_2032 |
| ESTHER : Roivainen_2004_J.Nucl.Med_45_2032 |
| PubMedSearch : Roivainen_2004_J.Nucl.Med_45_2032 |
| PubMedID: 15585478 |
| Title : Radiolabeled cholinesterase substrates: in vitro methods for determining structure-activity relationships and identification of a positron emission tomography radiopharmaceutical for in vivo measurement of butyrylcholinesterase activity - Snyder_2001_J.Cereb.Blood.Flow.Metab_21_132 |
| Author(s) : Snyder SE , Gunupudi N , Sherman PS , Butch ER , Skaddan MB , Kilbourn MR , Koeppe RA , Kuhl DE |
| Ref : Journal of Cerebral Blood Flow & Metabolism , 21 :132 , 2001 |
| Abstract : Snyder_2001_J.Cereb.Blood.Flow.Metab_21_132 |
| ESTHER : Snyder_2001_J.Cereb.Blood.Flow.Metab_21_132 |
| PubMedSearch : Snyder_2001_J.Cereb.Blood.Flow.Metab_21_132 |
| PubMedID: 11176278 |
| Title : Cholinesterases and the pathology of Alzheimer disease - Geula_1995_Alzheimer.Dis.Assoc.Disord_2_23 |
| Author(s) : Geula C , Mesulam MM |
| Ref : Alzheimer Disease & Associated Disorders , 2 :23 , 1995 |
| Abstract : Geula_1995_Alzheimer.Dis.Assoc.Disord_2_23 |
| ESTHER : Geula_1995_Alzheimer.Dis.Assoc.Disord_2_23 |
| PubMedSearch : Geula_1995_Alzheimer.Dis.Assoc.Disord_2_23 |
| PubMedID: 8534419 |