Meprylcaine
General
Type : Local anesthetic || Not A\/B H target
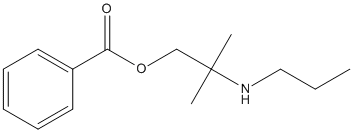
Chemical_Nomenclature : [2-methyl-2-(propylamino)propyl] benzoate
Canonical SMILES : CCCNC(C)(C)COC(=O)C1=CC=CC=C1
InChI : InChI=1S\/C14H21NO2\/c1-4-10-15-14(2,3)11-17-13(16)12-8-6-5-7-9-12\/h5-9,15H,4,10-11H2,1-3H3
InChIKey : VXJABHHJLXLNMP-UHFFFAOYSA-N
Other name(s) : Oracaine, Epirocaine, Epirocain, SCHEMBL24631, CHEMBL127810, CHEBI:93147, ZINC2040457
Target
Families : BCHE
References (6)
| Title : Inhibition of serotonin transporters by cocaine and meprylcaine through 5-TH2C receptor stimulation facilitates their seizure activities - Morita_2005_Brain.Res_1057_153 |
| Author(s) : Morita K , Hamamoto M , Arai S , Kitayama S , Irifune M , Kawahara M , Kihira K , Dohi T |
| Ref : Brain Research , 1057 :153 , 2005 |
| Abstract : Morita_2005_Brain.Res_1057_153 |
| ESTHER : Morita_2005_Brain.Res_1057_153 |
| PubMedSearch : Morita_2005_Brain.Res_1057_153 |
| PubMedID: 16125150 |
| Title : Chronic inhibition of the norepinephrine transporter in the brain participates in seizure sensitization to cocaine and local anesthetics - Arai_2003_Brain.Res_964_83 |
| Author(s) : Arai S , Morita K , Kitayama S , Kumagai K , Kumagai M , Kihira K , Dohi T |
| Ref : Brain Research , 964 :83 , 2003 |
| Abstract : Arai_2003_Brain.Res_964_83 |
| ESTHER : Arai_2003_Brain.Res_964_83 |
| PubMedSearch : Arai_2003_Brain.Res_964_83 |
| PubMedID: 12573515 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : The intravenous toxicity of local anesthetic agents in man - |
| Author(s) : Foldes FF , Davidson GM , Duncalf D , Kuwabara S |
| Ref : Clinical Pharmacology & Therapeutics , 6 :328 , 1965 |
| PubMedID: 14296030 |