Menthylacetate
General
Type : Aryl ester || Acetate
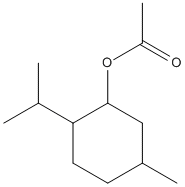
Chemical_Nomenclature : (5-methyl-2-propan-2-ylcyclohexyl) acetate
Canonical SMILES : CC1CCC(C(C1)OC(=O)C)C(C)C
InChI : InChI=1S\/C12H22O2\/c1-8(2)11-6-5-9(3)7-12(11)14-10(4)13\/h8-9,11-12H,5-7H2,1-4H3
InChIKey : XHXUANMFYXWVNG-UHFFFAOYSA-N
Other name(s) : Menthyl acetate, Menthol, acetate, Menthyl acetate racemic, (-)-Menthyl acetate, FEMA Number 2668, Neomenthyl acetate
Target
Families : Hormone-sensitive_lipase_like, Carb_B_Bacteria
References (4)
| Title : Engineering a Bacillus subtilis esterase for selective hydrolysis of d, l-menthyl acetate in an organic solvent-free system - Qiao_2023_RSC.Adv_13_10468 |
| Author(s) : Qiao J , Yang D , Feng Y , Wei W , Liu X , Zhang Y , Zheng J , Ying X |
| Ref : RSC Adv , 13 :10468 , 2023 |
| Abstract : Qiao_2023_RSC.Adv_13_10468 |
| ESTHER : Qiao_2023_RSC.Adv_13_10468 |
| PubMedSearch : Qiao_2023_RSC.Adv_13_10468 |
| PubMedID: 37021103 |
| Gene_locus related to this paper: 9baci-a0a2m8sv47 |
| Title : Efficient kinetic resolution of (+\/-)-menthol by a lipase from Thermomyces lanuginosus - De Yan_2017_Biotechnol.Appl.Biochem_64_87 |
| Author(s) : De Yan H , Li Q , Wang Z |
| Ref : Biotechnol Appl Biochem , 64 :87 , 2017 |
| Abstract : De Yan_2017_Biotechnol.Appl.Biochem_64_87 |
| ESTHER : De Yan_2017_Biotechnol.Appl.Biochem_64_87 |
| PubMedSearch : De Yan_2017_Biotechnol.Appl.Biochem_64_87 |
| PubMedID: 26549685 |
| Gene_locus related to this paper: humla-1lipa |
| Title : Biochemical studies on a versatile esterase that is most catalytically active with polyaromatic esters - Martinez-Martinez_2014_Microb.Biotechnol_7_184 |
| Author(s) : Martinez-Martinez M , Lores I , Pena-Garcia C , Bargiela R , Reyes-Duarte D , Guazzaroni ME , Pelaez AI , Sanchez J , Ferrer M |
| Ref : Microb Biotechnol , : , 2014 |
| Abstract : Martinez-Martinez_2014_Microb.Biotechnol_7_184 |
| ESTHER : Martinez-Martinez_2014_Microb.Biotechnol_7_184 |
| PubMedSearch : Martinez-Martinez_2014_Microb.Biotechnol_7_184 |
| PubMedID: 24418210 |
| Gene_locus related to this paper: 9bacl-h6nd87 |