MeJA
General
Type : Plant hormone
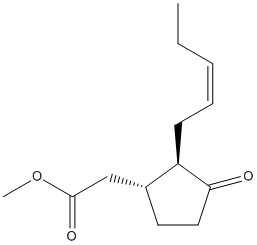
Chemical_Nomenclature : methyl 2-[(1R,2R)-3-oxo-2-[(Z)-pent-2-enyl]cyclopentyl]acetate
Canonical SMILES : CCC=CCC1C(CCC1=O)CC(=O)OC
InChI : InChI=1S\/C13H20O3\/c1-3-4-5-6-11-10(7-8-12(11)14)9-13(15)16-2\/h4-5,10-11H,3,6-9H2,1-2H3\/b5-4-\/t10-,11-\/m1\/s1
InChIKey : GEWDNTWNSAZUDX-WQMVXFAESA-N
Other name(s) : Methyl jasmonate, (-)-Methyl jasmonate, Methyl cis-jasmonate, Methyl (-)-jasmonate
Target
Families : Hydroxynitrile_lyase
References (7)
| Title : VvMJE1 of the grapevine (Vitis vinifera) VvMES methylesterase family encodes for methyl jasmonate esterase and has a role in stress response - Zhao_2016_Plant.Physiol.Biochem_102_125 |
| Author(s) : Zhao N , Lin H , Lan S , Jia Q , Chen X , Guo H , Chen F |
| Ref : Plant Physiol Biochem , 102 :125 , 2016 |
| Abstract : Zhao_2016_Plant.Physiol.Biochem_102_125 |
| ESTHER : Zhao_2016_Plant.Physiol.Biochem_102_125 |
| PubMedSearch : Zhao_2016_Plant.Physiol.Biochem_102_125 |
| PubMedID: 26934101 |
| Gene_locus related to this paper: vitvi-d7ssd8 |
| Title : Crystal structure of methylesterase family member 16 (MES16) from Arabidopsis thaliana - Li_2016_Biochem.Biophys.Res.Commun_474_226 |
| Author(s) : Li H , Pu H |
| Ref : Biochemical & Biophysical Research Communications , 474 :226 , 2016 |
| Abstract : Li_2016_Biochem.Biophys.Res.Commun_474_226 |
| ESTHER : Li_2016_Biochem.Biophys.Res.Commun_474_226 |
| PubMedSearch : Li_2016_Biochem.Biophys.Res.Commun_474_226 |
| PubMedID: 27109476 |
| Gene_locus related to this paper: arath-AT4G16690 |
| Title : Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice - Eduviere_2015_Life.Sci_132_20 |
| Author(s) : Eduviere AT , Umukoro S , Aderibigbe AO , Ajayi AM , Adewole FA |
| Ref : Life Sciences , 132 :20 , 2015 |
| Abstract : Eduviere_2015_Life.Sci_132_20 |
| ESTHER : Eduviere_2015_Life.Sci_132_20 |
| PubMedSearch : Eduviere_2015_Life.Sci_132_20 |
| PubMedID: 25921767 |
| Title : Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis - Yang_2008_Plant.Physiol_147_1034 |
| Author(s) : Yang Y , Xu R , Ma CJ , Vlot AC , Klessig DF , Pichersky E |
| Ref : Plant Physiol , 147 :1034 , 2008 |
| Abstract : Yang_2008_Plant.Physiol_147_1034 |
| ESTHER : Yang_2008_Plant.Physiol_147_1034 |
| PubMedSearch : Yang_2008_Plant.Physiol_147_1034 |
| PubMedID: 18467465 |
| Gene_locus related to this paper: arath-MES17 , arath-AT4G16690 , arath-AT4G37150 , arath-MES6 , arath-MES7 , arath-MES3 , arath-MES1 , arath-MES18 , arath-MES10 , arath-MES19 |
| Title : Methyl jasmonate-elicited herbivore resistance: does MeJA function as a signal without being hydrolyzed to JA? - Wu_2008_Planta_227_1161 |
| Author(s) : Wu J , Wang L , Baldwin IT |
| Ref : Planta , 227 :1161 , 2008 |
| Abstract : Wu_2008_Planta_227_1161 |
| ESTHER : Wu_2008_Planta_227_1161 |
| PubMedSearch : Wu_2008_Planta_227_1161 |
| PubMedID: 18214527 |
| Title : Cloning and expression of a tomato cDNA encoding a methyl jasmonate cleaving esterase - Stuhlfelder_2004_Eur.J.Biochem_271_2976 |
| Author(s) : Stuhlfelder C , Mueller MJ , Warzecha H |
| Ref : European Journal of Biochemistry , 271 :2976 , 2004 |
| Abstract : Stuhlfelder_2004_Eur.J.Biochem_271_2976 |
| ESTHER : Stuhlfelder_2004_Eur.J.Biochem_271_2976 |
| PubMedSearch : Stuhlfelder_2004_Eur.J.Biochem_271_2976 |
| PubMedID: 15233793 |
| Gene_locus related to this paper: lyces-MJE |
| Title : Differential expression of a novel gene in response to coronatine, methyl jasmonate, and wounding in the Coi1 mutant of Arabidopsis - Benedetti_1998_Plant.Physiol_116_1037 |
| Author(s) : Benedetti CE , Costa CL , Turcinelli SR , Arruda P |
| Ref : Plant Physiol , 116 :1037 , 1998 |
| Abstract : Benedetti_1998_Plant.Physiol_116_1037 |
| ESTHER : Benedetti_1998_Plant.Physiol_116_1037 |
| PubMedSearch : Benedetti_1998_Plant.Physiol_116_1037 |
| PubMedID: 9501136 |
| Gene_locus related to this paper: arath-clh1 |