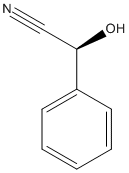
Mandelonitrile
General
Type : Cyanide || Benzaldehyde
Chemical_Nomenclature : (2S)-2-hydroxy-2-phenylacetonitrile
Canonical SMILES : C1=CC=C(C=C1)C(C#N)O
InChI : InChI=1S\/C8H7NO\/c9-6-8(10)7-4-2-1-3-5-7\/h1-5,8,10H\/t8-\/m1\/s1
InChIKey : NNICRUQPODTGRU-MRVPVSSYSA-N
Other name(s) : (S)-Mandelonitrile, (S)-2-Hydroxy-2-phenylacetonitrile, (-)-mandelonitrile, (S)-Benzaldehyde cyanohydrin, (S)-(-)-mandelonitrile
Target
Families : Hydroxynitrile_lyase
References (3)
| Title : Identical active sites in hydroxynitrile lyases show opposite enantioselectivity and reveal possible ancestral mechanism - Jones_2017_ACS.Catal_7_4221 |
| Author(s) : Jones BJ , Bata Z , Kazlauskas RJ |
| Ref : ACS Catal , 7 :4221 , 2017 |
| Abstract : Jones_2017_ACS.Catal_7_4221 |
| ESTHER : Jones_2017_ACS.Catal_7_4221 |
| PubMedSearch : Jones_2017_ACS.Catal_7_4221 |
| PubMedID: 28798888 |
| Gene_locus related to this paper: arath-HNL , hevbr-hnl |
| Title : Structural determinants of the enantioselectivity of the hydroxynitrile lyase from Hevea brasiliensis - Gartler_2007_J.Biotechnol_129_87 |
| Author(s) : Gartler G , Kratky C , Gruber K |
| Ref : J Biotechnol , 129 :87 , 2007 |
| Abstract : Gartler_2007_J.Biotechnol_129_87 |
| ESTHER : Gartler_2007_J.Biotechnol_129_87 |
| PubMedSearch : Gartler_2007_J.Biotechnol_129_87 |
| PubMedID: 17250917 |
| Gene_locus related to this paper: hevbr-hnl |
| Title : Structure determinants of substrate specificity of hydroxynitrile lyase from Manihot esculenta - Lauble_2002_Protein.Sci_11_65 |
| Author(s) : Lauble H , Miehlich B , Forster S , Kobler C , Wajant H , Effenberger F |
| Ref : Protein Science , 11 :65 , 2002 |
| Abstract : Lauble_2002_Protein.Sci_11_65 |
| ESTHER : Lauble_2002_Protein.Sci_11_65 |
| PubMedSearch : Lauble_2002_Protein.Sci_11_65 |
| PubMedID: 11742123 |
| Gene_locus related to this paper: manes-hnl |